Session Information
Date: Monday, October 22, 2018
Title: 4M102 ACR Abstract: Misc Rheum & Inflam DZ I: DADA2, Cardiac Sarcoid,Cancer Immunotherapy(1911–1916)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Rheumatic immune-related adverse events (irAEs) from PD-1 inhibitor immune checkpoint immunotherapy can not only lead to cessation of immunotherapy, but also can be disabling and can also persist beyond cessation. Currently no biomarker can predict rheumatic irAEs. However, it is known that oncological response to PD-1 inhibitor therapy is predicted by pre-treatment neutrophil to lymphocyte ratio (NLR) and given that rheumatic irAEs are strongly associated with an oncological response to PD-1 inhibitor therapy, it therefore stands to reason that rheumatic irAEs and NLR prior to therapy might be associated, though this is currently unknown.
Methods: We examined the relationship between NLR and the development of rheumatic irAEs in all patients first treated with a PD-1 inhibitor in our center prior to January 1, 2017, as identified by hospital pharmacy dispensing records. Patients were included if a NLR could be calculated from a complete blood count performed at the institutional laboratory within two weeks prior to commencing treatment; this was subsequently log-transformed. The relationship between NLR and the development of rheumatic irAEs was assessed using the two-tailed Fisher’s Exact Test, with NLR cut-point determined using a recursive partitioning algorithm. Treatment efficacy was examined using oncological response, with responders defined as those who achieved at least partial response. The relationship between oncological response and NLR was also examined using identical methodology. Analysis was performed using R 3.5.0 and the rpart package.
Results: Out of the 211 patients included in the study, 15 developed a rheumatic irAE. The median age was 66 years and median treatment duration was 19 months. The primary cancer was melanoma in 91 patients (41%) and non-small cell lung cancer in 82 patients (39%). Nivolumab was used in 127 patients (57%). The log-NLR cut-point of 1.7 (untransformed = 5.5), as determined by recursive partitioning, is comparable to similar cut-points used in previous studies. Using this cut-point, there was a statistically significant association between lower log-NLR and the development of rheumatic irAEs (p = 0.007). At the same cut-point, there was also a statistically significant relationship between lower log-NLR and oncological response to therapy (p < 0.001).
Conclusion: Lower pre-treatment NLR prospectively predicts the development of rheumatic irAEs in our cohort, using methodology comparable to previous studies demonstrating the relationship between NLR and oncological response. Its promise as a biomarker for rheumatic irAEs should be explored in larger cohorts.
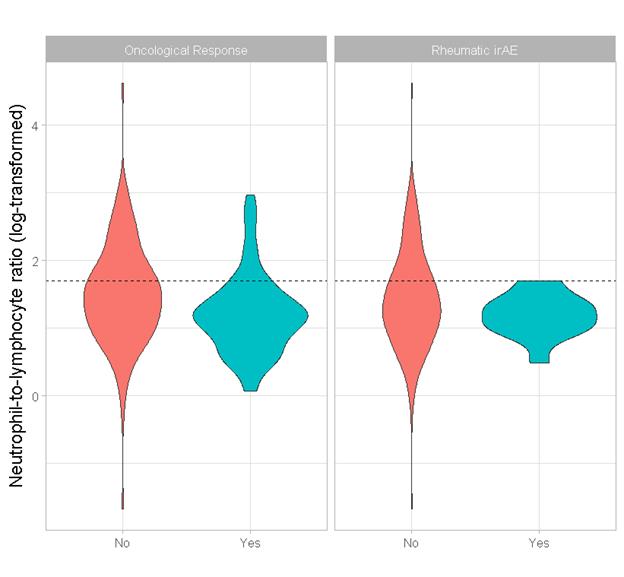
Figure 1. Violin plots of log-NLR. Left: oncological responders (blue) vs. non-responders (red). Right: experiences a rheumatic irAE (blue) vs. did not experience a rheumatic irAE (blue).
To cite this abstract in AMA style:
McMaster C, Liew D, Shamdasani P, Leung J, Frauman A, Cebon J, Buchanan R. Neutrophil-to-Lymphocyte Ratio Prospectively Predicts the Development of Rheumatic Immune-Related Adverse Events from PD-1 Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/neutrophil-to-lymphocyte-ratio-prospectively-predicts-the-development-of-rheumatic-immune-related-adverse-events-from-pd-1-inhibitor-therapy/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-to-lymphocyte-ratio-prospectively-predicts-the-development-of-rheumatic-immune-related-adverse-events-from-pd-1-inhibitor-therapy/
