Session Information
Date: Monday, October 22, 2018
Title: Patient Outcomes, Preferences, and Attitudes Poster I: Patient-Reported Outcomes
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Golimumab has shown clinical efficacy and tolerability within its clinical trial program. No systematic
outcome data regarding patient‐reported outcomes and health economic parameters reflecting real-world use of golimumab in Austria are currently available. Methods:
Go Active is a prospective, non‐interventional, multi‐center study in Austria. The impact of
golimumab therapy on work productivity and activity (WPAI) and quality of life (RAQoL for RA
patients, AsQoL for axSpA patients, PsAQoL for PsA patients) is assessed by using patient-reported outcomes. Patients (target recruitment: n = 220) are followed up to 2 years. In this interim analysis (data cut-off: 03 May 2018) changes in WPAI and QoL from baseline to month 3 are analyzed after recruitment was completed. Results:
At total of 234 patients were enrolled in the study and 189 patients were included in this analysis (81 patients with RA, 56 patients with axSpA, and 52 patients with PsA).
Median age at registration was 52 years (patients with RA: 56 years, patients with axSpA: 40 years, and patients with PsA: 53.5 years). Almost two thirdsof patients were female (81% of patients with RA, 39% patients with axSpA, and 58% of patients with PsA). Most patients were biological-naïve at study entry (78% of all patients, 75% of patients with RA, 79% of patients with axSpA, and 83% of patients with PsA). 38% of patients were not employed (54% of patients with RA, 25% of patients with axSpA and SpA); 14% due to incapacity for work (11% of patients with RA, 21% of patients with axSpA, and 15% of patients with SpA) and 55% due to age-related pension (61% of patients with RA, 21% of patients with axSpA, and 69% of patients with SpA). Most of the patients, who worked for a fee, worked full time.
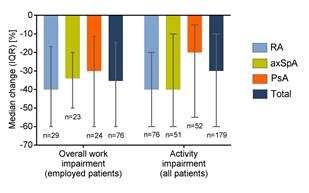
179 of all patients and 92 of employed patients completed the WPAI questionnaire at baseline and after 3 months. Overall work productivity improved by 35% (40% for patients with RA, and 34% for patients with axSpA and 30% for patients with PsA) and activity impairment by 30% (40% for patients with RA and axSpA, and 20% for patients with PsA; Fig. 1). Quality of life scores improved by 7.5 for patients with RA, by 5 for patients with axSpA, and by 3 for patients with PsA.
Fig. 1: WPAI questionnaire – changes from baseline after 3 months of golimumab treatment
This interim analysis shows that golimumab is an effective treatment for patients with RA, axSpA and PsA and leads to an improvement of work productivity and daily activities as well as of quality of life already within the first 3 months of treatment. At study entry, most patients were biological-naïve and employed.
To cite this abstract in AMA style:
Dejaco C, Mueller T, Zamani MD O, Kurtz MD U, Egger MD S, Resch Passini MD J, Totzauer MD A, Eisterer W, Yazdani-Biuki MD Univ.Doc. B, Schwingenschloegl MD T, Peichl MD. Univ.Doc. Msc P, Kraus A, Naerr PhD G, Rickert MD. MBA V. Golimumab Improves Work Productivity and Activity As Well As Quality of Life in Patients with Rheumatoid Arthritis (RA), Psoriasis Arthritis (PsA) and Axial Spondyloarthritis (axSpA): Interim Results from a Non-Interventional Study in Austria (Go Active) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/golimumab-improves-work-productivity-and-activity-as-well-as-quality-of-life-in-patients-with-rheumatoid-arthritis-ra-psoriasis-arthritis-psa-and-axial-spondyloarthritis-axspa-interim-results/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/golimumab-improves-work-productivity-and-activity-as-well-as-quality-of-life-in-patients-with-rheumatoid-arthritis-ra-psoriasis-arthritis-psa-and-axial-spondyloarthritis-axspa-interim-results/