Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Secukinumab versus Adalimumab for the Treatment of Ankylosing Spondylitis: A Cost per Responder Analysis from Korean Perspective
Dam Kim1, Hyojin Kim2, SeongHa Cho2, Min-Chan Park1
1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
2Patient Access Team, Novartis Korea, Seoul, Korea
Background/Purpose:
Various kinds of biologic agents are available for treating ankylosing spondylitis(AS). However, there is considerable economic burden for patients and society. This study was performed to estimate the response rate of secukinumab (fully human anti-interleukin 17 A) and adalimumab in AS patients, and to compare the cost-effectiveness between secukinumab and adalimumab from Korean perspective.
Methods:
A systematic literature search was performed via PubMed for relevant randomized controlled trials (RCTs) for response rate. The cost per responder for each treatment was estimated by dividing drug acquisition cost for the treatment course with its response rate of Assessment of Spondyloarthritis International Society(ASAS) outcomes. Response rates in anti-TNF-naive subjects were extracted from RCTs of secukinumab and adalimumab, respectively. All analyses were calculated in case of both with or without loading condition of secukinumab. Cost was expressed with US dollars (USD) (1 USD = 1,082 Korean Won).
Results:
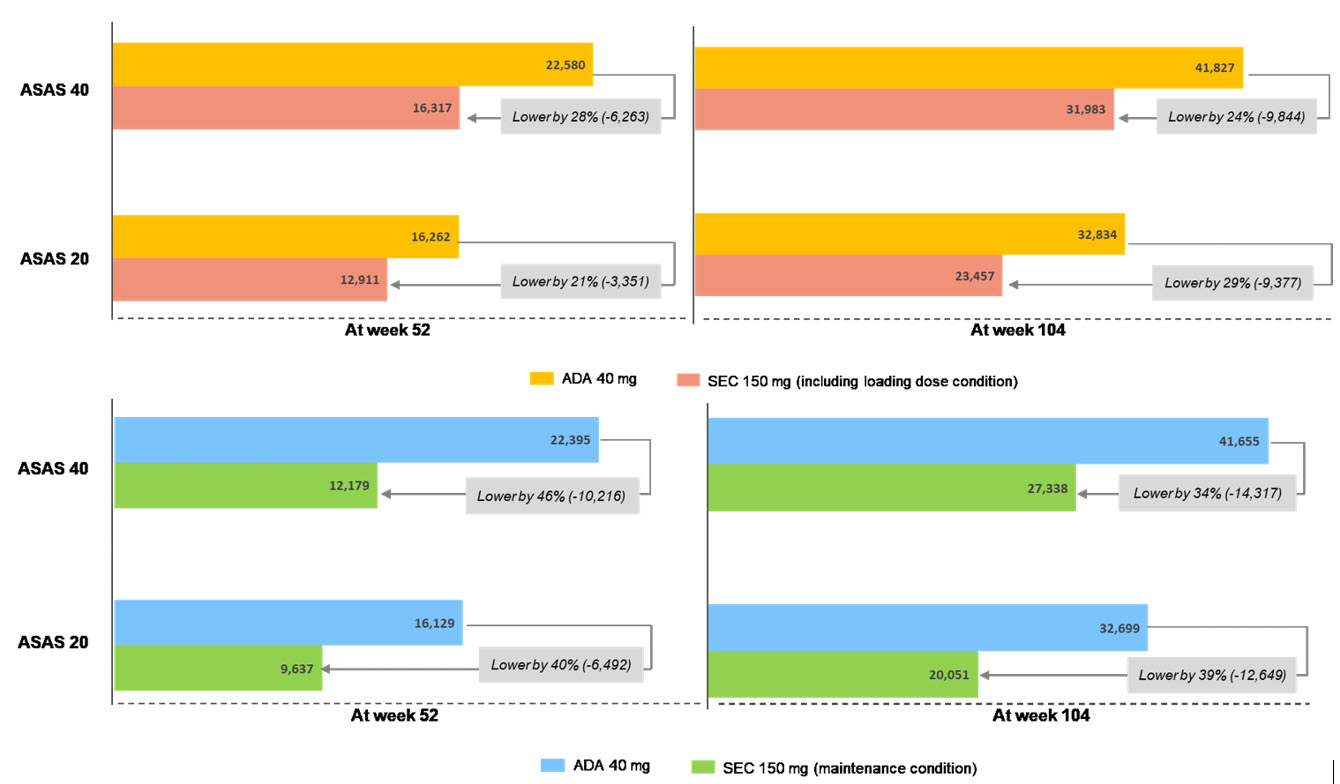
Of the 295 articles retrieved, five RCTs were identified including long-term response data. The ASAS 20 and 40 response rates from selected studies were comparable between secukinumab and adalimumab. The cost per ASAS 20 responder were lower by 40% in secukinumab compared with adalimumab: USD 9,637 vs. USD 16,129 at 52 weeks and USD 20,051 vs. USD 32,699 at 104 weeks for secukinumab vs. adalimumab (in case of maintenance condition), respectively. In addition, the cost per ASAS 40 responder were also lower by about 40% in secukinumab: USD 12,179 vs. USD 22,395 at 52 weeks and USD 27,338 vs. USD 41,655 for secukinumab vs. adalimumab, respectively. Even in case of loading condition with secukinumab at 52 and 104 weeks, secukinumab showed lower cost per responder by approximately 25% than adalimumab.
Conclusion:
The response rates of secukinumab and adalimumab were comparable. The costs per responder for ASAS 20 and 40 were consistently lower for secukinumab compared with adalimumab. The treatment with secukinumab for biologic-naive AS patients could be the cost-effective treatment option in Korea with a given budget.
Figure 1. Cost per response of ASAS 40 and 20 for secukinumab (SEC) vs. adalimumab (ADA) for the treatment of AS at week 52 and 104 (upper: with SEC loading condition, bottom: with SEC maintenance condition) (unit: USD)
To cite this abstract in AMA style:
Kim D, Kim H, Cho S, Park MC. Secukinumab Versus Adalimumab for the Treatment of Ankylosing Spondylitis: A Cost per Responder Analysis from Korean Perspective [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/secukinumab-versus-adalimumab-for-the-treatment-of-ankylosing-spondylitis-a-cost-per-responder-analysis-from-korean-perspective/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-versus-adalimumab-for-the-treatment-of-ankylosing-spondylitis-a-cost-per-responder-analysis-from-korean-perspective/