Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Osteoarthritis (OA) treatment is typically administered to control symptoms. Diacerein (DIA) reduces interleukin (IL)-1 receptor on chondrocytes and prevents structural degradation of joint tissue. Direct injection of DIA into joint is preferred to increase its bioavailability and reduce systemic side effects. We investigated whether poly(d,l–lactide-co-glycolide) (PLGA) nanoparticles (NPs) could effectively deliver DIA and inhibit the inflammatory reaction.
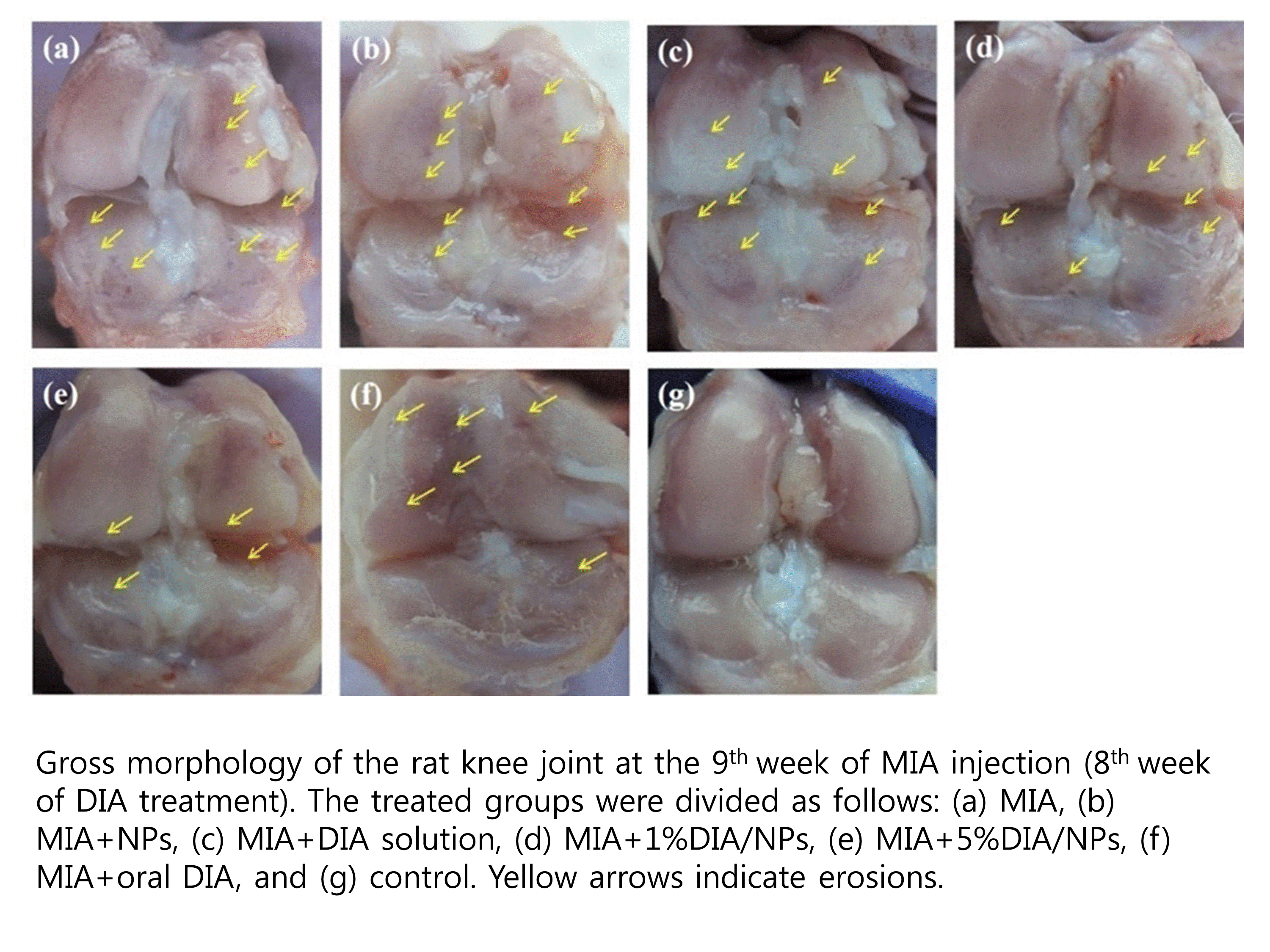
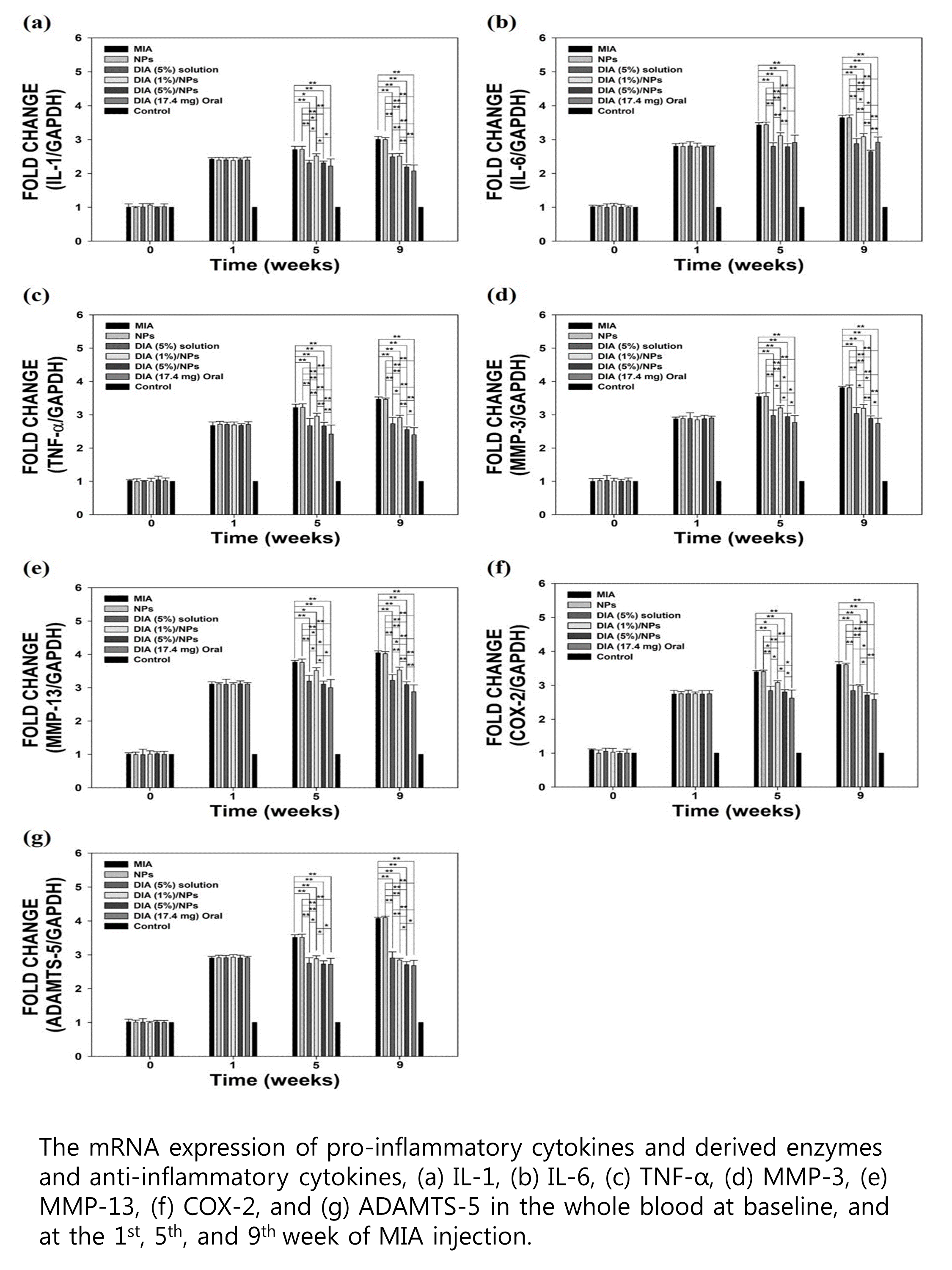
Methods: Monosodium iodoacetate (MIA)-induced OA rats were divided into seven groups with injection of MIA alone (group 1) and with, NPs (2), 5% DIA (3), 1% DIA/NPs (4) or 5% DIA/NPs (5), oral DIA (6), and non-treated healthy control rats (7). The mRNA expression of pro-inflammatory cytokines and derived enzymes were investigated, including interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-¥á), matrix metalloproteinase (MMP)-3, MMP-13, cyclo-oxygenase (COX)-2, and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5. Macroscopic, radiographic, and histologic evaluations of the joints were done at 8 weeks of DIA treatment.
Results: In group 5, the mRNA expression of inflammatory cytokines and derived enzymes decreased gradually and significantly compared with groups 1–4. Group 3 levels of mRNA expression were decreased at 5 weeks similar to that of group 5, but increased at 9 weeks. Group 6 displayed the lowest mRNA levels of inflammatory cytokines and derived enzymes, except for IL-6, which was lowest in group 5. Macroscopic erosions were fewest in group 5, followed in order by groups 6, 4, and 3. Micro-computed tomography revealed only slight irregularity of the joint surface and minimal erosion of articular cartilage in group 5 and 6.
Conclusion: Intra-articular injection of DIA/NPs inhibited the inflammatory reaction and progression in OA, with histologically less cartilage damage detected than induced by oral DIA. The 5% DIA/NPs dose produced significant reductions of the serum IL-6 levels compared with oral administration, and is a candidate treatment for the inhibition of inflammation and protection of the cartilage in OA.
To cite this abstract in AMA style:
Jung JH, Choi SJ, Song GG, Kim SE, Kim HJ. Comparison of Oral Administration of Diacerein, Intra-Articular Injection of Diacerein Solution, and Intra-Articular Injection of Diacerein-Loaded Nanoparticle in Osteoarthritis Rat Model [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/comparison-of-oral-administration-of-diacerein-intra-articular-injection-of-diacerein-solution-and-intra-articular-injection-of-diacerein-loaded-nanoparticle-in-osteoarthritis-rat-model/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-oral-administration-of-diacerein-intra-articular-injection-of-diacerein-solution-and-intra-articular-injection-of-diacerein-loaded-nanoparticle-in-osteoarthritis-rat-model/
