Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Point of care tailored treatment strategies are of value to improve rheumatoid arthritis (RA) patient outcomes. Musculoskeletal ultrasound (MSUS) is increasingly used as a modality by which rheumatologists diagnose and monitor the progression of RA. The multi-biomarker disease activity (MBDA) blood test is a commercially available assay that measures twelve inflammatory biomarkers to score RA disease activity on a scale of 1-100. This pilot study evaluated whether baseline MSUS and MBDA scores or their early changes, are predictive of 12-week clinical response in RA patients treated with tofacitinib.
Methods: 25 RA patients who met entry criteria (including baseline disease activity score using ESR [DAS28] >3.2 and power Doppler US [PDUS] score >10) were treated with open-label tofacitinib at the approved dose of 5 mg PO BID and assessed at baseline, 2 weeks and 12 weeks. MSUS was performed at each visit scoring 34-joints for PDUS and GSUS. Other metrics examined were MBDA score, clinical disease activity index (CDAI), disease activity score (DAS28). Associations between MBDA score/PDUS/GSUS at baseline or their changes from baseline to 2 or 12 weeks, and the change in DAS28/CDAI from baseline to 12 weeks, were assessed using Pearson correlation coefficients.
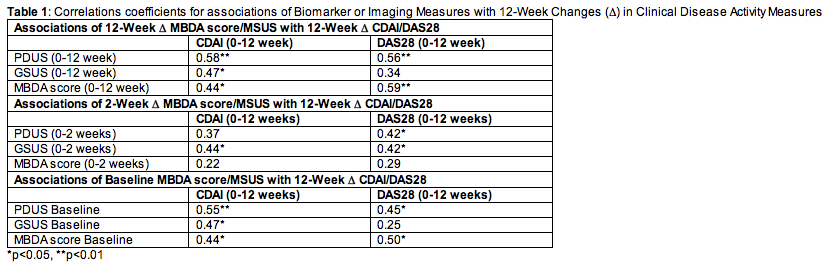
Results: Mean age was 52 years, mean disease duration 10.4 years, 88% of patients were female, 40% Caucasian, and 96% were RF/CCP positive. At baseline, the mean (SD) DAS28 was 6.26 (1.2) and CDAI was 40 (13.2). There was significant improvement in PDUS, GSUS, MBDA score, DAS28, and CDAI over 12 weeks (all p<0.0001). Correlations of 12-week changes in MBDA score or MSUS with 12-week changes in CDAI or DAS28 were all significant (correlation coefficients 0.44-0.58), except for GSUS with DAS28 (Table 1). Changes from baseline to 2 weeks in PDUS or GSUS were associated with significant DAS28 response at 12 weeks (Table 1). Baseline PDUS, GSUS and MBDA score were significant predictors of 12-week CDAI or DAS28 responses, except for GSUS with DAS28 response (Table 1).
Conclusion: This study showed that RA patients treated with tofacitinib for 12 weeks demonstrated significant responses with clinical, imaging, and biomarker end-points. In addition, baseline PDUS and MBDA score were predictive of the DAS28 and CDAI response at 12 weeks. This is the first study to evaluate early MSUS and MBDA changes as predictors of clinical response in RA patients treated with tofacitinib.
To cite this abstract in AMA style:
Razmjou A, Brook J, Kaeley G, Elashoff D, Ranganath VK. Baseline Power Doppler and Multi-Biomarker Disease Activity Score Predict 12-Week Disease Activity Response to Tofacitinib [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/baseline-power-doppler-and-multi-biomarker-disease-activity-score-predict-12-week-disease-activity-response-to-tofacitinib/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-power-doppler-and-multi-biomarker-disease-activity-score-predict-12-week-disease-activity-response-to-tofacitinib/