Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Sprifermin, a truncated form of human fibroblast growth factor 18 (FGF18), is being investigated as a potential cartilage and disease-modifying OA drug. In vitro, Sprifermin induces chondrocyte proliferation and extracellular matrix (ECM) production. Here, we characterized the ECM remodeling of human knee OA articular cartilage in response to long-term Sprifermin treatment.
Methods: Full depth articular cartilage explants from OA patients (npatients=11) were cultured for 70 days. Freshly prepared media with either Sprifermin (900, 450, 225 ng/mL), FGF18 (450 ng/mL), IGF-1 (100 ng/mL, positive control) or placebo formulation (negative control) was added once weekly (nexplants/treatment/patient=2). Metabolic activity was measured weekly by AlamarBlue. Biomarkers of ECM remodelling were quantified in conditioned media using well-described ELISAs; exPro-C2 reflecting type II collagen formation and exAGNx1 reflecting aggrecanase-mediated aggrecan degradation. Data were baseline-corrected and normalized to placebo for each individual patient and reported as mean ± standard errors of the mean (SEM).
Results: In human OA articular cartilage cultured ex vivo, the positive control IGF-1 induced ProC2 and increased metabolic activity in all patient explants as well. All investigated doses of Sprifermin and FGF18 continuously increased metabolic activity compared to placebo over the 70 days of treatment (fig. 1A). The aggrecan degradation biomarker exAGNx1 was increased by the investigated doses of Sprifermin and FGF18 as compared to placebo (fig. 1B). exAGNX1 release peaked at 21 and then gradually decreased in presence of Sprifermin and FGF18, indicating a shift of a catabolic to an anabolic phenotype as cause of treatment. Type II collagen formation, measured by exProC2, was initially (day 7-28) decreased by Sprifermin and FGF18 compared to placebo, but from day 35 to 70 exProC2 increased in a dose dependent manner, with sprifermin superior to FGF-18 (fig. 1C). The increase in exProC2 release from Sprifermin (900 ng/mL)-treated explants were 1.5-fold of placebo at day 49.
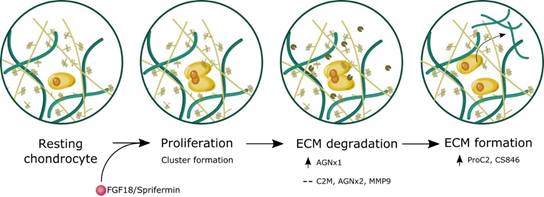
Conclusion: The data indicate that human knee OA articular cartilage explants increase their metabolic activity and formation of type II collagen in response to long-term treatment with Sprifermin and FGF18. The induction of cartilage formation is preceded by cartilage degradation, indicating that a catabolic process is needed to make room of proliferation of chondrocyte and renewal of cartilage (fig. 2).
Figure 2. Schematic illustration of sprifermin effects on articular cartilage
To cite this abstract in AMA style:
Reker D, Thudium CS, Siebuhr AS, Gantzel T, Ladel C, Michaelis M, Karsdal MA, Gigout A, Bay-Jensen AC. Articular Cartilage from Osteoarthritis Patients Shows Extracellular Matrix Remodeling over the Course of Treatment with Sprifermin (Recombinant human fibroblast growth factor 18) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/articular-cartilage-from-osteoarthritis-patients-shows-extracellular-matrix-remodeling-over-the-course-of-treatment-with-sprifermin-recombinant-human-fibroblast-growth-factor-18/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/articular-cartilage-from-osteoarthritis-patients-shows-extracellular-matrix-remodeling-over-the-course-of-treatment-with-sprifermin-recombinant-human-fibroblast-growth-factor-18/