Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: EBV infects B cells and is associated with several autoimmune diseases, including RA. Prior studies have shown that patients with active classified RA have altered immune responses to EBV, including elevated anti-viral capsid antigen (VCA) antibodies that indicate recent reactivation. We hypothesized that the abnormal response to EBV occurs in the preclinical period and contributes to the initial development of autoimmunity in subjects who develop RA.
Methods: Serum from military subjects who developed RA (n=83) and controls matched for age, race, sex, region, and timing of blood draw (n=83) was obtained from Department of Defense Serum Repository as previously described (1). All RA subjects met 1987 ACR revised criteria for RA or were diagnosed by a board-certified rheumatologist. Sera were tested for anti-EBNA-1 IgG (2), anti-VCA IgG (Wampole, Cranbury, NJ), and anti-CCP3.1 and RF IgM (both Inova, San Diego, CA) by ELISA. Statistical analysis was by Chi square, t-test, and ANOVA. A mixed model compared EBV levels at one-year intervals followed by 1 month intervals to identify the time when levels differed significantly between cases and controls (P<0.05). Multiple comparison adjustment with a stepdown Holm-simulated method was used to control the family-wise type I error rate.
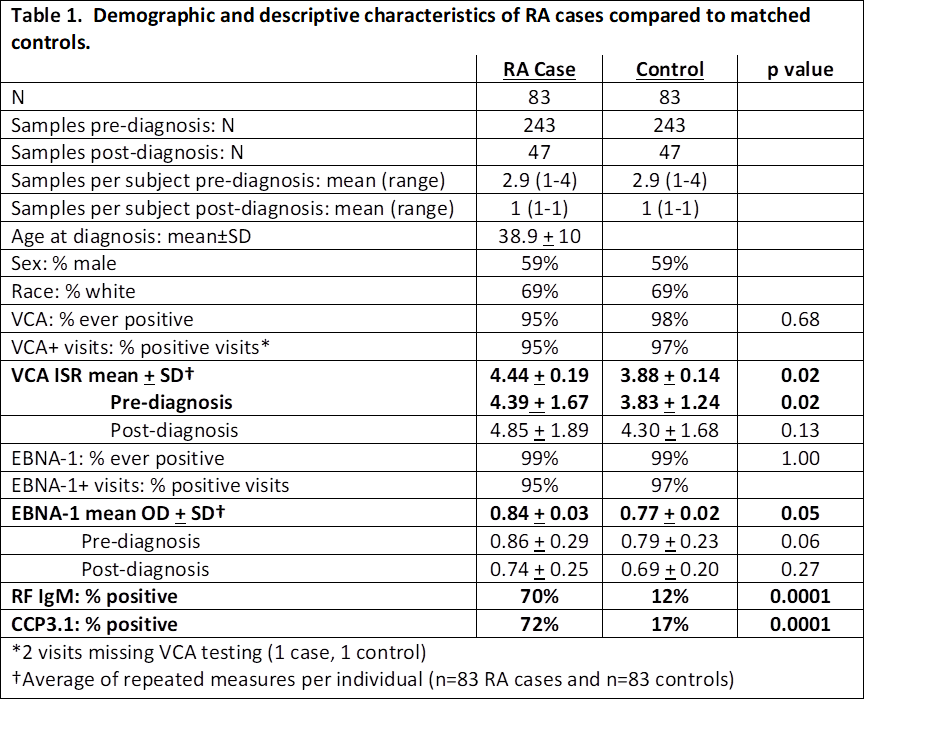
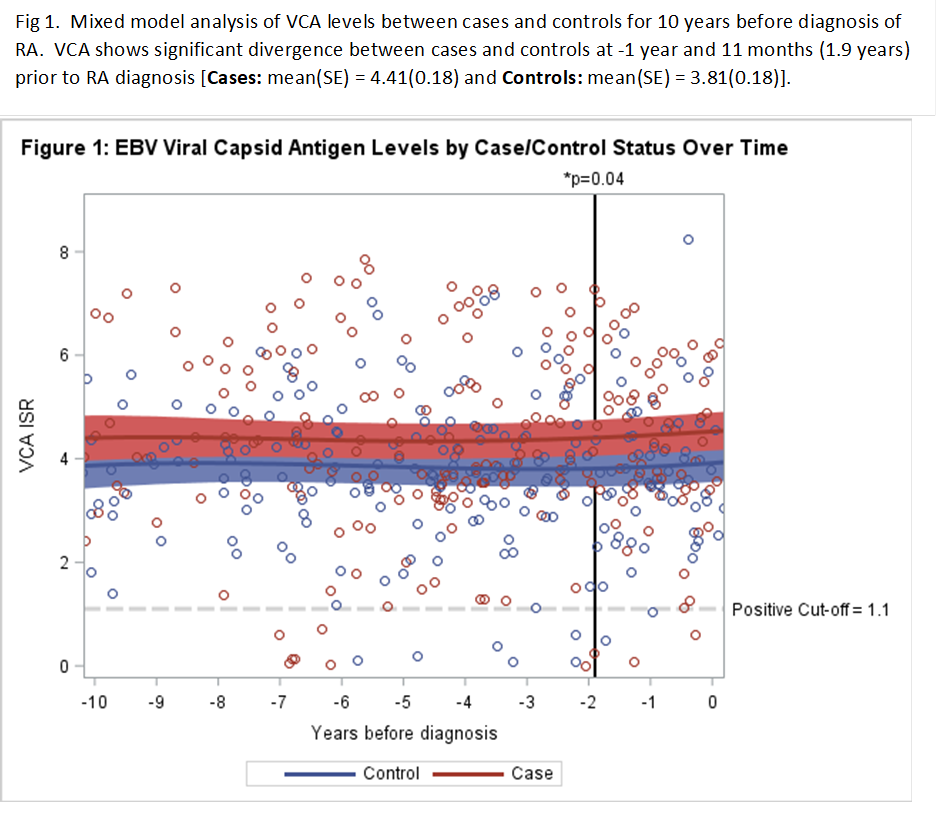
Results: Levels of VCA and EBNA-1 antibodies were significantly higher in RA cases vs controls over all visits, most notably in the pre-diagnosis period (Table 1). Analysis of EBV antibody levels in the 10 years prior to diagnosis showed VCA diverged 1.9 years before onset RA compared to controls (Fig 1), and EBNA-1 diverged at 3.5 years.
Conclusion: An altered immune response to EBV characterized by relatively elevated EBNA-1 and VCA antibodies is seen in pre-clinical RA. Elevated anti-VCA antibodies suggests that viral reactivation may contribute to the future development of RA. Further studies are needed to assess the complex lifecycle of EBV and immune regulation of viral infection in the pre-clinical period and the role in promoting RA-related autoantibody and classified disease development.
References
1. Majka DS, et al. Ann Rheum Dis. 2008; 67(6):801-7.
2. McClain MT et al. Arthritis Rheum 2006; 54(1):360-8.
The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the authors, DoD, or any component agency. The views expressed in this presentation are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
To cite this abstract in AMA style:
Berens HM, Bemis EA, Demoruelle MK, Harley JB, James JA, Edison J, Deane KD, Norris JM, Holers VM. Altered Serologic Responses to Epstein Barr Virus Precede Clinical Disease Development in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/altered-serologic-responses-to-epstein-barr-virus-precede-clinical-disease-development-in-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/altered-serologic-responses-to-epstein-barr-virus-precede-clinical-disease-development-in-rheumatoid-arthritis/