Session Information
Date: Sunday, October 21, 2018
Title: B Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
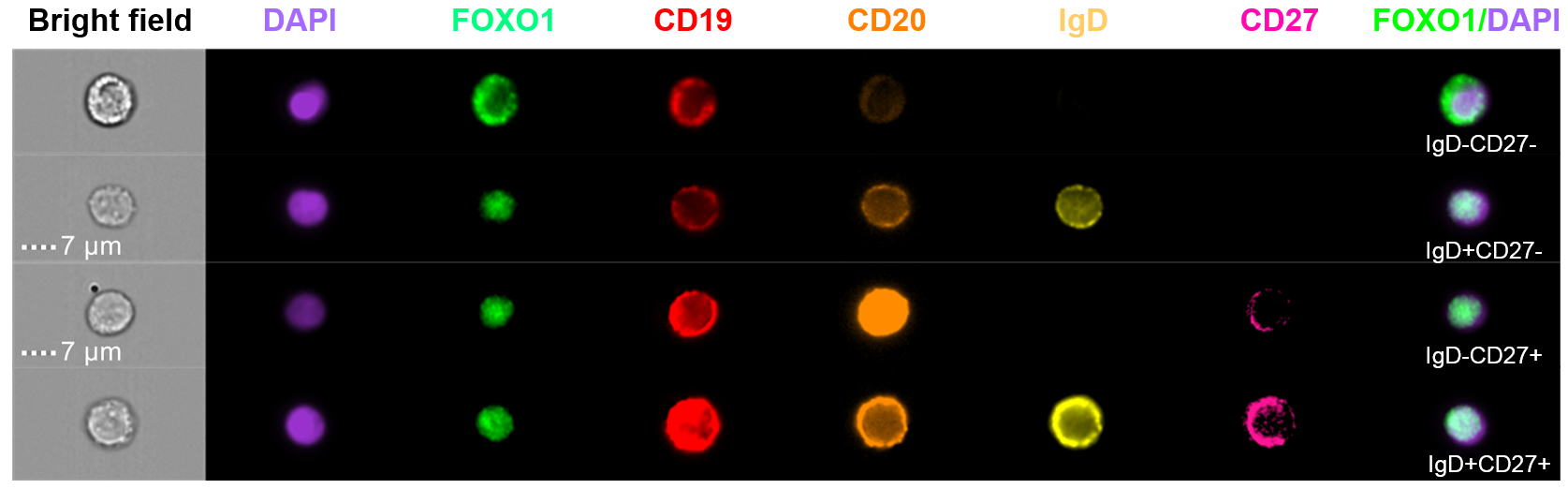
Background/Purpose: A key integrator of external stimuli in lymphocytes, Forkhead box protein O1 (FOXO1) is a highly attractive factor to study in SLE because it is central to B cell activation/maturation and integration of metabolic and inflammatory stimuli. When active, it remains in the nucleus, but upon Akt phosphorylation downstream of T or B Cell Receptor signaling, FOXO1 is inactivated and shuttles to the cytoplasm, linking FOXO1 localization to function. In SLE, both T and B cells are hyperactive, and respond more quickly and strongly to antigen, producing a disproportionate inflammatory response. Thus, we hypothesized that SLE lymphocytes would have altered FOXO1 localization, reflecting altered lymphocyte activation.
Methods: To address this hypothesis, we first developed a method of examining dynamic native FOXO1 localization in human peripheral lymphocyte subsets using imaging flow cytometry (IFC). IFC combines the quantitative power of flow cytometry with the qualitative images of microscopy and can be performed with many fewer cells than are needed for the more traditional methods. We demonstrated that we can visualize native FOXO1 and detect significant kinetic differences in localization within user-defined subsets of primary peripheral human T and B cells. We then used IFC to compare FOXO1 localization in SLE and healthy donor lymphocytes.
Results: Most T and B cell subsets demonstrated nuclear FOXO1 localization in both healthy controls and individuals with SLE. However, FOXO1 was significantly more cytoplasmic in SLE in atypical memory IgD-CD27- B cells (see representative figure below). Cytoplasmic-predominant FOXO1 (CytoFox) B cells were significantly increased in SLE patients as compared to healthy controls, and the levels of CytoFox B cells correlated positively with SLE disease activity. The highest abundance of CytoFox B cells was observed in African American females with SLEDAI ≥ 6 and elevated anti-dsDNA Antibodies or lupus nephritis. The phenotype of CytoFox B cells in SLE included relatively low CD20 expression and high granularity/side scatter.
Conclusion: We report, here, on dramatic cytoplasmic localization of FOXO1 in IgD-CD27- (atypical memory) B cells. So-called “Double Negative” (DN) B cells have previously been shown to be increased in SLE and enriched in autoreactive clones. As FOXO1 phosphorylation downstream of B cell receptor-dependent signaling is required for nuclear exclusion, CytoFox B cells likely represent a high state of B cell activation with excess signaling and/or loss of phosphatase activity. We hypothesize that CytoFox B cells in lupus represent a novel biomarker for the expansion of pathologic, autoreactive B cells which may provide new insights into the pathophysiology of SLE.
To cite this abstract in AMA style:
Hritzo M, Golding A. Cytoplasmic FOXO1 Identifies Novel Disease-Activity Associated B Cell Subsets in SLE [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/cytoplasmic-foxo1-identifies-novel-disease-activity-associated-b-cell-subsets-in-sle/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cytoplasmic-foxo1-identifies-novel-disease-activity-associated-b-cell-subsets-in-sle/