Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: MMF suppresses immune function by inhibiting T cell dependent and independent humoral immune responses. This study investigates the humoral immune response to the PPSV23 vaccine by MMF in a larger sample size. Furthermore, this study explores the effect of MMF dose and concurrent steroid use on the anti-pneumococcal antibody responses.
Methods: In this observational cross-sectional study, patients treated with immune suppressive medications who had received PPVS23 were identified and stratified based on receiving MMF or non-MMF immunosuppressants (control group). The humoral response was assessed in both groups using serum IgG titers against 14 pneumococcal polysaccharides (ELISA) as a surrogate marker. The stimulation index (SI) was calculated by dividing the post by the pre immunization titer, which was compared between the MMF and non-MMF groups .The primary endpoints of the study were protective titers of >1.3 µg/ml or either a 4-fold, 3-fold, or 2-fold increase in the SI for 70% of the 14 pneumococcal polysaccharides.
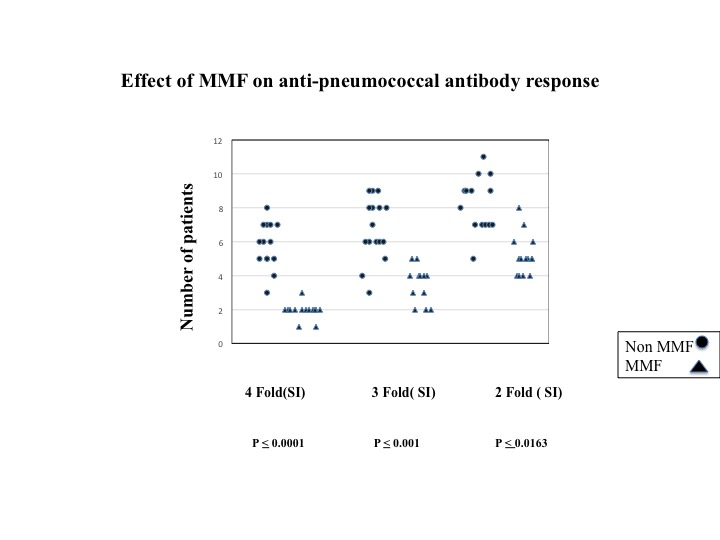
Results: The total study subjects included 39 patients .The MMF group included 23 patients: 21 with SLE, 1 with uveitis, and 1 with DM. The control group included 16 patients: 12 with SLE, 2 with RA, 1 with uveitis, and 1 with PsA. The humoral responses in the MMF group were significantly lower compared to the control group. 40 % of patients in the MMF group vs. 60 % of the control group had protective antibody levels (> 1.3 ug/ml). However, statistically significant differences were only observed in serotypes 51 and 4 (p = 0.05 & p = 0.025). Suppressed antibody responses were observed in the MMF group as defined by a lack of a 4-fold (p = 0.0001), 3-fold (p = 0.001), and 2-fold (p = 0.0163) increase in the SI vs. the control group. 20 %(n=3) of the study group and 50% (n=8) in the control group had more than a 2-fold increase in post-immunization antibody titers to 10 of the 14 serotypes. Patients receiving more than 1.5 g of MMF daily had more suppressed antibody responses defined as a lack of a 4-fold increase (p = 0.04) in the SI compared to patients taking less than 1.5 g of MMF daily. Patients in both the MMF and control groups received less than 10 mg of prednisone that had no effect on the anti-pneumococcal antibody responses.
Conclusion: This data suggests that humoral responses to pneumococcal polysaccharides are more blunted in patients receiving MMF compared to other immunosuppressants. There was a dose effect of MMF on the humoral response. However, no effect of steroids was observed. Evaluation of post-vaccination humoral immunity should be considered in patients receiving MMF.
To cite this abstract in AMA style:
Prakash P, Tratenberg M, Bobic S, Zhang R, Sperber K, Wasserman A, Ash J. Effects of Mycophenolate Mofetil (MMF) on Immunogenicity of Ppsv-23 Vaccine in Patients with Systemic Lupus Erythematosus and Other Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/effects-of-mycophenolate-mofetil-mmf-on-immunogenicity-of-ppsv-23-vaccine-in-patients-with-systemic-lupus-erythematosus-and-other-autoimmune-diseases/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-mycophenolate-mofetil-mmf-on-immunogenicity-of-ppsv-23-vaccine-in-patients-with-systemic-lupus-erythematosus-and-other-autoimmune-diseases/