Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Juvenile myositis (JM) can worsen quality of life (QoL) via proximal weakness, rashes, and treatments side effects. QoL legacy instruments may be limited by floor effects, unresponsiveness, and cost, justifying the need for new approaches. We present cross-sectional data for initial validation of Patient-Reported Outcomes Measurement Information System (PROMIS) in JM.
Methods:
Children (5-17 yo) with JM and parents enrolled at routine clinic visits. Demographic/clinical data were recorded, e.g. Physician Global Assessment of Disease Activity (PGA); Disease Activity Score (DAS-Total, -Muscle, -Skin); Childhood Myositis Assessment Scale (CMAS); muscle enzymes; nailfold capillary end row loops (NFC-ERL); neopterin; absolute NK cell count; C4 level. Children (8-17 yo) and parents (of all patients 5-17yo) completed patient/parent-proxy versions of PedsQL-generic core scales and rheumatology module (PedsQL-GC, -RM) and PROMIS Pain Interference, Physical Function (Mobility, Upper Extremity), Fatigue, and Emotional Distress (Depressive Symptoms, Anxiety) fixed short forms. Interrater (i.e. patient-parent) reliability was assessed via intraclass correlation coefficients (ICCs). Concurrent and construct validity were assessed via SpearmanÕs correlation coefficients between PROMIS and PedsQL scales and between PROMIS domains and clinical/lab data respectively.
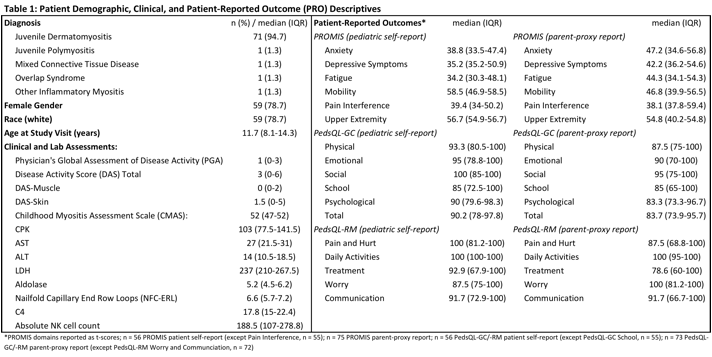
Results:
Table 1 lists descriptive data. Patient-Parent ICCs were highest for PedsQL-GC (>0.6 for all except Social domain [=0.48]) compared with PedsQL-RM and PROMIS (>0.4 for all but PedsQL-RM Worry/Communication domains). Patient/parent PROMIS domains demonstrated concurrent validity (SpearmanÕs >0.4) with related PedsQL-GC/-RM domains (Table 2). Pediatric PROMIS and PedsQL-GC/-RM did not correlate with clinical/lab data; parent-proxy PROMIS fatigue, physical function (mobility, upper extremity), and multiple PedsQL-GC/-RM domains correlated with DAS-muscle and CMAS (Table 3).
Conclusion:
PROMIS pediatric fixed short forms demonstrate similar evidence for reliability and validity compared with PedsQL-GC/-RM in children with JM. Patient and parent-proxy reports differ sufficiently to suggest both should be collected. PROMIS can be considered for clinical/research use in JM.
To cite this abstract in AMA style:
Ardalan K, Cella D, Pachman LM, Gray EL, Lee J, Fahey K, Wolfe M, Curran ML, Marques MC, Chang RW. Initial Validation of Patient-Reported Outcomes Measurement Information System (PROMIS®) in Children with Juvenile Myositis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/initial-validation-of-patient-reported-outcomes-measurement-information-system-promis-in-children-with-juvenile-myositis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initial-validation-of-patient-reported-outcomes-measurement-information-system-promis-in-children-with-juvenile-myositis/