Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Myositis autoantibody testing is now widely commercially available, with an evolving role in routine clinical care. However, the use and performance of commercial "myositis panels" in usual practice have been poorly studied. We examined commercial myositis autoantibody panel ordering patterns and testing results in a large tertiary-care health system.
Methods:
We conducted a retrospective observational study of all adult patients with a commercial myositis autoantibody panel ordered at all outpatient and two inpatient locations of the University of Pennsylvania between January 2011 and March 2016. We abstracted information about patient demographics, myositis panel orders [specialty of the ordering provider, symptoms/signs which prompted the order (indication), performing commercial vendor, autoantibodies tested], and relevant clinical, laboratory, radiographic, and histopathologic data. For each subject, clinical diagnosis was assigned considering all available information except autoantibody profile. Bohan and Peter’s and Sontheimer’s criteria were used for PM/DM and clinically amyopathic dermatomyositis (CADM), with positive skin biopsy required for definite CADM. Equivocal cases were resolved by consensus among all investigators.
Results:
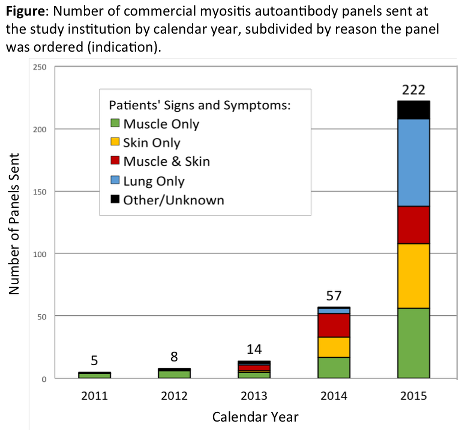
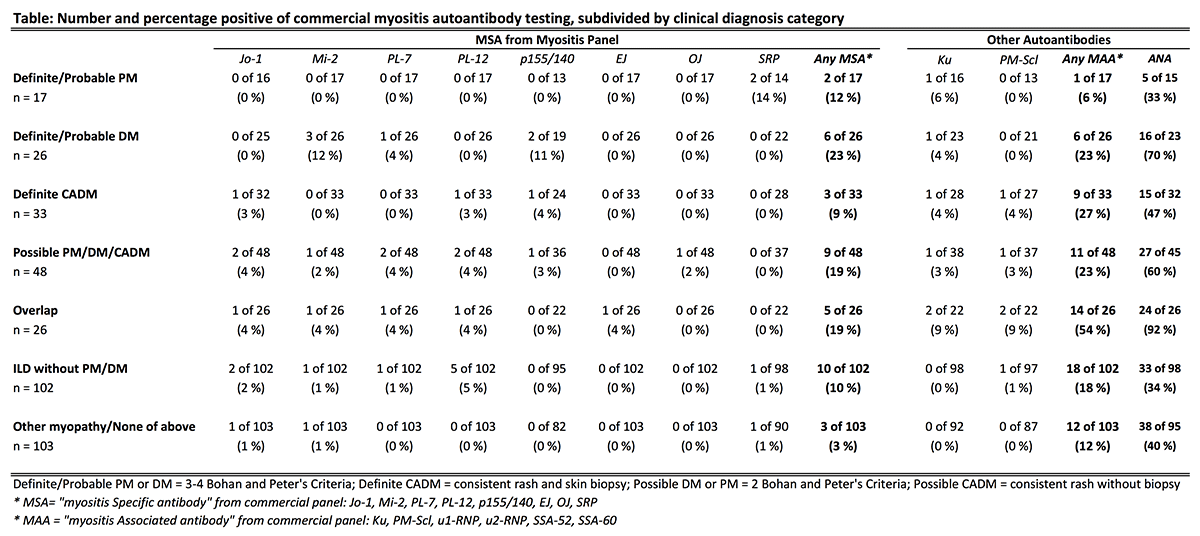
378 patients were included (66% women, mean age 55 ± 15 years). Myositis panels were ordered most often by rheumatology (39%), pulmonology (23%), dermatology (18%), and inpatient medicine (12%). ARUP Laboratories performed 72% of testing. The number of myositis panels ordered markedly increased over the study period for all indications (Figure) and departments. 10.3% of subjects had positive myositis specific autoantibodies (MSA); 19.6% had positive myositis associated autoantibodies (MAA). The rate of positive MSA was similar for all indications [p=0.47]. Only 11 of 76 (14.5%) of patients with probable/definite PM, DM, or CADM had positive MSA (Table). A comparable rate of positive MSA occurred in patients with ILD without myositis [10/102 (9.8%); p=0.34]. Patterns for MAA followed similar trends.
Conclusion:
Commercial myositis autoantibody panel testing has dramatically increased for expanding clinical indications by rheumatologists and non-rheumatologists alike. Clinicians must be aware that negative MSA testing with these assays was common, even in patients with clinically affirmed disease. MSA were found at a similar rate in patients with isolated ILD, and may provide important information for this population. Continued study of the performance of commercial myositis autoantibody testing is required as the role of these assays further develops.
To cite this abstract in AMA style:
Gandiga* PC, Zhang* J, Thomas P, Werth VP, Sangani S, Kolasinski SL, George MD. Clinical Utilization Patterns and Performance of Commercial Myositis Autoantibody Panels in Routine Practice [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/clinical-utilization-patterns-and-performance-of-commercial-myositis-autoantibody-panels-in-routine-practice/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-utilization-patterns-and-performance-of-commercial-myositis-autoantibody-panels-in-routine-practice/