Session Information
Date: Monday, November 6, 2017
Title: Plenary Session II
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: It is not known whether TNF blockers can be stopped in non-radiographic (nr-axSpA) patients who are in remission. In ABILITY-1, adalimumab (ADA) significantly improved clinical response vs placebo (PBO) in patients (pts) with nr-axSpA. ABILITY-3 (NCT01808118), reported here, assessed if ADA can be discontinued or should be continued in nr-axSpA pts in sustained remission after a 28-wk open-label period.
Methods: ABILITY-3 enrolled adult pts diagnosed with nr-axSpA, fulfilling ASAS criteria but NOT modified New York criteria who had objective evidence of active MRI inflammation in the SI joints or spine or elevated high-sensitivity CRP at screening, active disease at baseline (ASDAS ≥2.1, BASDAI ≥4, total back pain ≥4), and inadequate response to ≥2 NSAIDs. Pts who achieved ASDAS inactive disease (ASDAS <1.3) with open-label ADA 40 mg every other wk at wk 16, 20, 24, and 28 were randomized to 40-wk, double-blind PBO (withdrawal) or ADA (continuation) in period 2. The primary efficacy endpoint was the proportion of patients who did not experience a flare (ASDAS ≥2.1 at 2 consecutive study visits) during period 2. Secondary endpoints were also assessed up to wk 68 (nonresponder imputation).
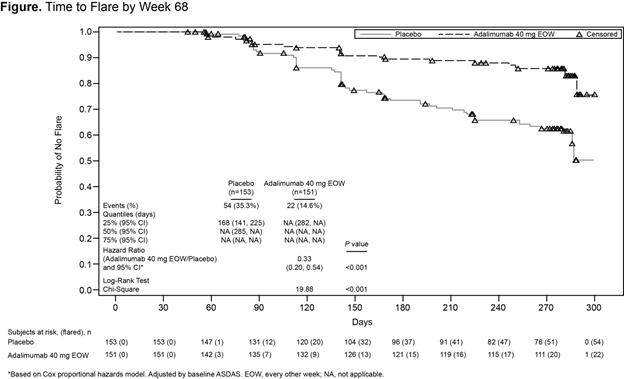
Results: Of 673 enrolled pts, 305 (45%) were randomized to double-blind treatment (Table). A significantly greater proportion of patients treated with ADA vs PBO had no flares (70% vs 47%; P<0.001) at wk 68; the relative risk of flare with treatment withdrawal was 1.77. Time to flare analysis showed significantly lower risk of flare for ADA vs PBO (Figure). At wk 68, significantly greater proportions of ADA vs PBO patients achieved secondary endpoints, except for HAQ-S (Table). Among pts who received ADA at any time, 77% reported adverse events (AEs) and 4% reported a serious AE; nasopharyngitis (17%), upper respiratory tract infection (12%), worsening of axSpA (9%), headache (8%), and diarrhea (6%) were the most common. During period 2, incidence of AEs was similar for ADA and PBO (65% vs 69%), incidence of serious AEs was higher for PBO vs ADA (7% vs 1%), and the most common AEs in both the ADA and PBO groups were nasopharyngitis (16% vs 13%), upper respiratory tract infection (13% vs 8%), and worsening of axSpA (6% vs 14%; none serious).
|
Table. Baseline Characteristics and Efficacy Outcomes at Week 68
|
|||
|
Variable Baseline, mean (SD)* |
Adalimumab (40 mg EOW) n=152 |
Placebo n=153 |
P Value |
|
Age, y |
34.7 (10.3) |
35.3 (10.2) |
0.611 |
|
Female, n (%) |
56 (37) |
60 (39) |
0.724 |
|
HLA-B27 positive, n (%) |
132 (87) |
134 (88) |
0.866 |
|
SpA symptom duration, y |
6.4 (6.9) |
7.1 (6.8) |
0.358 |
|
SpA duration from diagnosis, y |
1.9 (2.9) |
1.8 (2.9) |
0.711 |
|
ASDAS |
3.5 (0.9) |
3.5 (0.8) |
0.851 |
|
BASDAI |
6.8 (1.4) |
6.8 (1.5) |
0.851 |
|
Total back pain |
7.0 (1.7) |
7.0 (1.8) |
0.946 |
|
ASDAS, wk 28 |
0.7 (0.4) |
0.6 (0.4) |
0.355 |
|
Wk 68†, n (%) |
|
||
|
No flare |
106 (70) |
72 (47) |
<0.001 |
|
ASDAS ID |
87 (57) |
51 (33) |
<0.001 |
|
ASDAS MI |
89 (59) |
49 (32) |
<0.001 |
|
ASDAS CII |
102 (67) |
69 (45) |
<0.001 |
|
ASAS20 |
107 (70) |
72 (47) |
<0.001 |
|
ASAS40 |
100 (66) |
70 (46) |
<0.001 |
|
ASAS 5/6 |
87 (57) |
49 (32) |
<0.001 |
|
ASAS PR |
64 (42) |
41 (27) |
0.005 |
|
BASDAI50 |
103 (68) |
72 (47) |
<0.001 |
|
Change from baseline in BASFI‡, LSmean ± SE |
–3.97±0.11 n=111 |
–3.51±0.13 n=77 |
0.007 |
|
Change from baseline in HAQ-S‡, LSmean ± SE |
–0.68±0.04 n=112 |
–0.58±0.04 n=79 |
0.088 |
|
ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CII, clinically important improvement; EOW, every other week; HAQ-S, Health Assessment Questionnaire for the Spondyloarthropathies; HLA-B27, human leukocyte antigen B27; ID, inactive disease; LS, least squares; MI, major improvement; PR, partial remission; SE, standard error; SpA, spondyloarthritis. *Baseline characteristics at overall study baseline (wk 0) unless indicated otherwise. †Nonresponder imputation for wk 68 values; only data prior to rescue included. ‡Mixed effect Model Repeat Measurement. P value using analysis of variance (baseline) or 2-sided Pearson chi-square test (wk 68 vs lead-in open-label baseline). P value for HAQ-S and BASFI change from baseline using analysis from 2-sided repeated measures model with fixed effect of treatment. |
|||
Conclusion: In pts with nr-axSpA who achieved sustained remission with ADA, continued therapy was associated with significantly more patients maintaining remission and lower disease activity than treatment withdrawal. These results support the continuation of ADA therapy after achievement of sustained remission. Safety findings were consistent with the established safety profile of ADA.
To cite this abstract in AMA style:
Landewé RBM, Sieper J, Mease PJ, Inman RD, Wang X, Li M, Pangan AL, Anderson JK. Efficacy and Safety of Continuing Versus Withdrawing Adalimumab in Maintaining Remission in Patients with Non-Radiographic Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-continuing-versus-withdrawing-adalimumab-in-maintaining-remission-in-patients-with-non-radiographic-axial-spondyloarthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-continuing-versus-withdrawing-adalimumab-in-maintaining-remission-in-patients-with-non-radiographic-axial-spondyloarthritis/