Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
No data are available from head-to-head randomized controlled trials (RCTs) between secukinumab 150mg (SEC; an anti-IL-17A) and golimumab 50mg (GOL; a TNF inhibitor [TNFi]) in patients with active AS. Matching-Adjusted Indirect Comparison (MAIC) is a method supported and used in decision-making by UK National Institute for Health and Care Excellence (NICE). It ensures comparisons across effectively balanced trial populations. This study assessed the comparative effectiveness of SEC and GOL up to week 24 using MAIC with pooled individual patient data (IPD) from the RCTs MEASURE 1 (M1) and MEASURE 2 (M2) and published aggregate data from GO-RAISE.
Methods:
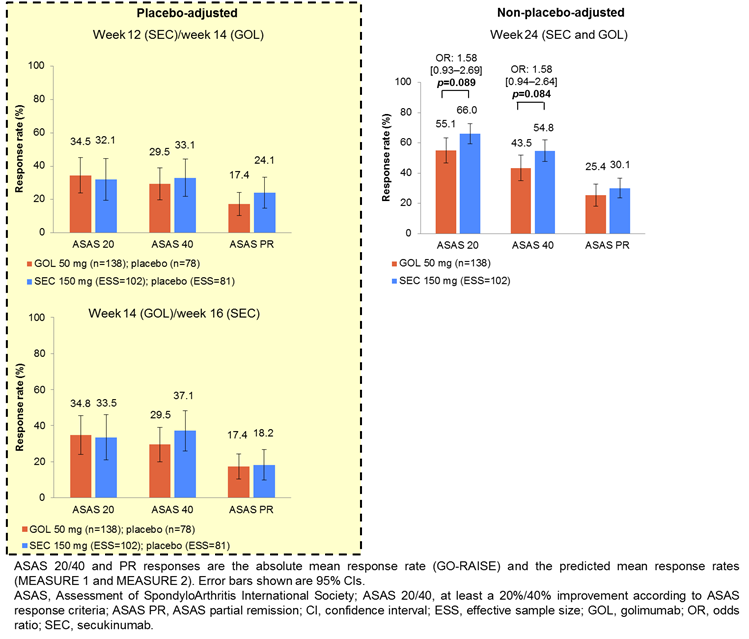
IPD from the pooled SEC arms of M1 and M2 (n=197) were weighted to match the baseline characteristics of the GOL arm from GO-RAISE (n=138). Pooled data were used to maximize the effective sample size (ESS) for SEC. Placebo arms were also matched; placebo-adjusted comparisons were possible only until week 16 because patients could receive active treatment from this time onwards. Logistic regression determined weights for age, sex, BASFI, disease duration, CRP and previous TNFi therapy. Variables were selected by expert opinion and regression analyses. Recalculated outcomes from M1, M2 (SEC, ESS=102; placebo, ESS=81) were compared with GO-RAISE (GOL, n=138; placebo, n=78). Pairwise comparisons – reported as odds ratios (ORs [95% CIs]) – were performed for Assessment of SpondyloArthritis international Society (ASAS) 20, 40 and partial remission (PR) responses at nearest-equivalent time points across trials: weeks 12 (SEC)/14 (GOL), 14 (GOL)/16 (SEC) and 24 (SEC and GOL). Strict thresholds were avoided when interpreting p values in line with American Statistical Association 2016 guidance.
Results:
There was no evidence of differences in ASAS 20 and 40 responses between SEC and GOL at weeks 12/14 and 14/16 (both placebo-adjusted). At week 24, non-placebo-adjusted ASAS 20 and 40 responses were higher with SEC than GOL (OR [95% CI]: 1.58 [0.93–2.69] p=0.089 and 1.58 [0.94–2.64] p=0.084, respectively). There was no evidence of differences in ASAS PR responses between SEC and GOL at weeks 12/14, 14/16 and 24. A sensitivity analysis that also matched for BASDAI score yielded similar results.
Conclusion:
There was no evidence of differences in ASAS responses between SEC and GOL in placebo-adjusted analyses. In non-placebo-adjusted analyses, SEC showed higher ASAS 20 and 40 responses than GOL at week 24.
To cite this abstract in AMA style:
Maksymowych WP, Choy E, Yazici Y, Walsh JA, Thom H, Kalyvas C, Fox T, Gandhi K, Jugl S. Comparative Effectiveness of Secukinumab and Golimumab in Ankylosing Spondylitis: Assessed By Matching-Adjusted Indirect Comparison Using Pivotal Phase 3 Clinical Trial Data [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-and-golimumab-in-ankylosing-spondylitis-assessed-by-matching-adjusted-indirect-comparison-using-pivotal-phase-3-clinical-trial-data/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-and-golimumab-in-ankylosing-spondylitis-assessed-by-matching-adjusted-indirect-comparison-using-pivotal-phase-3-clinical-trial-data/