Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pregnancies in women with active rheumatic disease often result in poor neonatal outcomes. Prior data suggests that hydroxychloroquine (HCQ) can reduce disease activity and flares in pregnant women with systemic lupus. Due to significant increases in the volume of distribution and renal clearance, we hypothesize that HCQ levels decrease during pregnancy, thereby increasing the risk for worsened maternal disease activity and thus poor neonatal outcomes. We tested the relationship between HCQ levels and maternal/neonatal outcomes in a prospective pregnancy cohort.
Methods: We enrolled pregnant patients with rheumatic disease from a single center on a stable HCQ dose between 2013-2016 into an observational study. Frozen serum samples were sent to NMS laboratories and analyzed using high performance liquid chromatography/tandem mass spectrometry. HCQ levels were defined as non-therapeutic (<100 ng/mL) or therapeutic (≥100 ng/mL). Categorical outcomes were analyzed using Fisher’s Exact Test and continuous outcomes were analyzed using linear regression models.
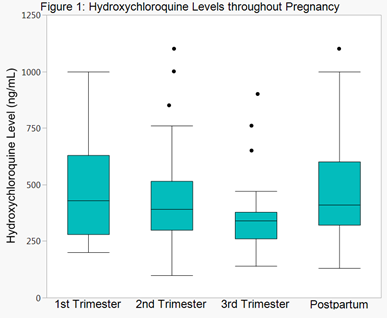
Results: 50 patients were enrolled in the study; 28 (56%) had systemic lupus, 7 (14%) had rheumatoid arthritis, and the remainder had undifferentiated connective tissue disease or other rheumatic diseases. Concomitant medications included corticosteroids in 14 (28%) and azathioprine in 9 (18%). Median HCQ levels for all patients at the most common dose (400 mg) trended down throughout pregnancy and returned near baseline post-partum (p=0.08) (Figure 1, excluding levels <100 ng/mL). Of all patients, 24% had at least one visit with HCQ levels < 100 ng/mL (29% SLE and 18% non-SLE). Excluding one outlier, mean physician global assessment (PGA) scores in lupus patients decreased by 0.07 per 100 ng/mL increase in HCQ level (p=0.04). There was no relationship between HCQ levels and C3, C4, or double-stranded DNA antibody in lupus patients. When analyzed by HCQ level closest to delivery, 100% of lupus patients with HCQ levels <100 ng/mL delivered premature (n=4), whereas only 24% of individuals with levels ≥100 ng/mL delivered prematurely (n=21), (p=0.01). In non-lupus patients, there was no difference in prematurity by HCQ level category.
Conclusion: In this small cohort of patients, HCQ levels declined as pregnancy progressed, suggesting a physiologic effect of pregnancy on drug levels and the potential need to optimize dosing. Lupus PGA scores improved with increasing HCQ levels. All lupus patients with HCQ levels <100 ng/mL delivered prematurely, suggesting a role for therapeutic drug monitoring.
Acknowledgements: This work was supported by funding from the Derfner Foundation.
To cite this abstract in AMA style:
Balevic S, Cohen-Wolkowiez M, Eudy AM, Schanberg LE, Clowse MEB. Hydroxychloroquine Level Decreases throughout Pregnancy: Implications for Maternal and Neonatal Outcomes [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/hydroxychloroquine-level-decreases-throughout-pregnancy-implications-for-maternal-and-neonatal-outcomes/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-level-decreases-throughout-pregnancy-implications-for-maternal-and-neonatal-outcomes/