Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
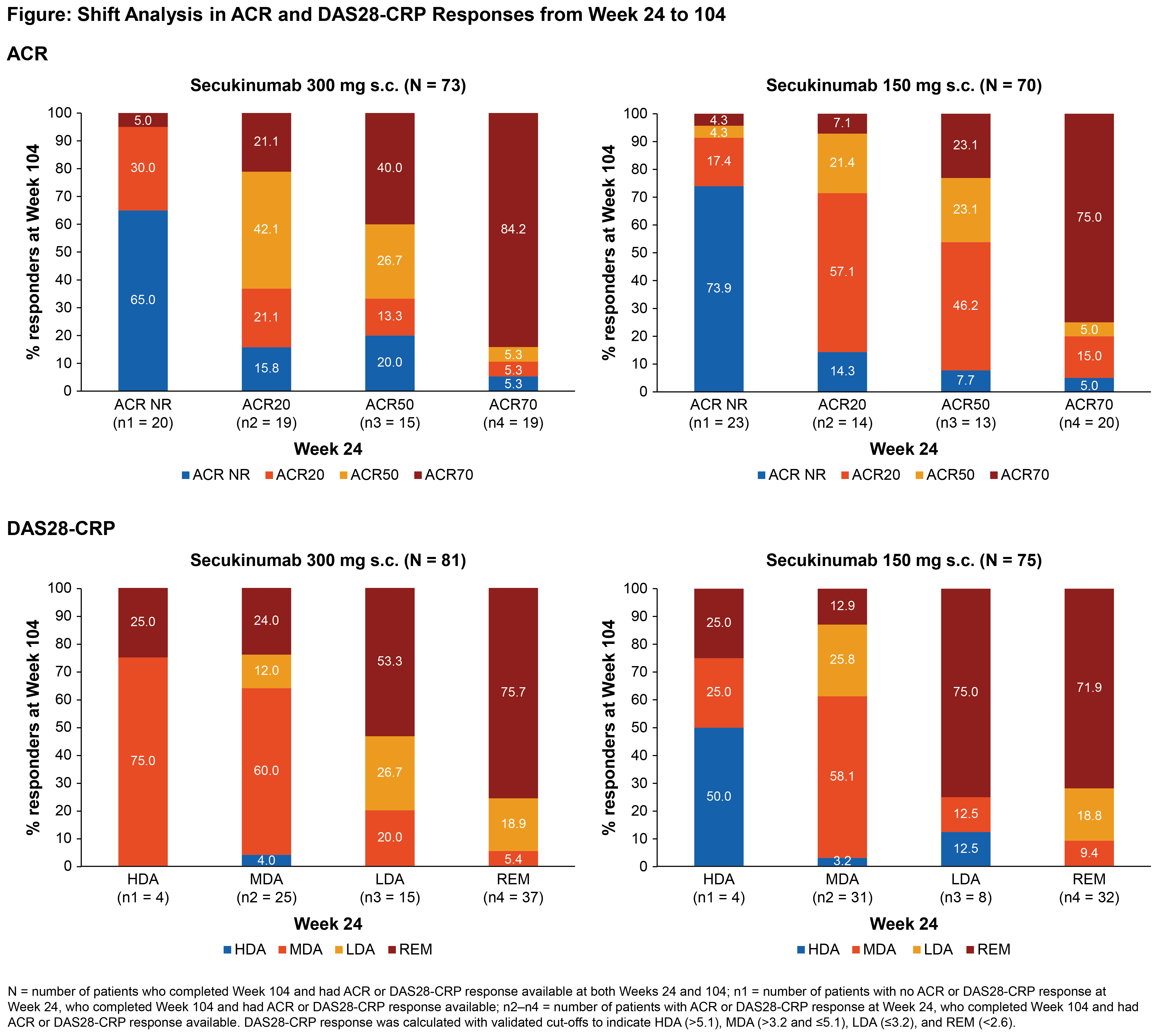
Background/Purpose: Achieving, sustaining, and improving clinical responses to biologics in PsA are important parts of EULAR and GRAPPA recommendations aimed to optimize treatment goals.1,2 Here we present patient (pt)-level secukinumab data and report the likelihood of improving, sustaining, or worsening an ACR response and disease status (28-joint disease activity score using CRP [DAS28-CRP]) from Week (Wk) 24 to 104 in pts with active PsA from the FUTURE 2 trial.3,4
Methods: Data from FUTURE 2 trial through Wk 104 have been previously reported.4 This post-hoc shift analysis was performed on ACR responses (ACR non-responders [NR], ACR20, 50, or 70) between Wks 24 and 104 for subgroups of secukinumab-treated pts categorized by their highest ACR response criteria at the earlier time point, by evaluating whether this response was improved, sustained or worsened at the later time point, using mutually exclusive ACR response categories and as observed analyses. Similar shift analyses on DAS28-CRP derived criteria were performed in 4 exclusive categories: high, moderate, and low disease activity (HDA, MDA, and LDA), and remission (REM) only.5
Results: In total, 86/100 (86%) and 76/100 (76%) pts in the secukinumab 300 and 150 mg groups, respectively, completed the 104-wk treatment. Of which, 73/70 and 81/75 pts in secukinumab 300/150 mg were eligible for ACR and DAS28-CRP shift analysis, respectively, from Wk 24 to 104. Baseline demographics and clinical characteristics were balanced across treatment groups.3,4 Most secukinumab-treated pts who achieved at least an ACR20, 50, or 70 response at Wk 24, improved or sustained their response at Wk 104 (Figure). Similarly, a majority of pts who were in the MDA, LDA, or REM status at Wk 24 sustained or improved their disease status related to DAS28-CRP score at Wk 104 (Figure). In secukinumab 300 mg group, 53% of pts with LDA improved to REM and a majority (76%) of pts with REM maintained their status from Wk 24 to 104, whereas, in 150 mg group, a majority of pts (75% and 72% with LDA and REM, respectively) improved or maintained their status by Wk 104.
Conclusion: In this post-hoc analysis, a majority of secukinumab-treated pts who achieved at least ACR20 response or at least MDA at Wk 24 sustained or improved their ACR response or disease status at Wk 104. Numerically higher sustained ACR response and LDA or REM rate was observed for secukinumab 300 mg, thereby extending the sustainability of ACR response and lowering the disease activity that has been previously reported at group level.2,3
References: 1. Gossec L, et al. Ann Rheum Dis 2016;75:499–510; 2. Coates LC, et al. J Rheumatol 2014;41:1237–9; 3. McInnes IB, et al. Lancet 2015;386:1137–46; 4. McInnes IB, et al. Arthritis Rheumatol 2016;68;abstract: 2757; 5. Wells G, et al. Ann Rheum Dis 2009;68:954–60.
To cite this abstract in AMA style:
Emery P, McInnes IB, Mease PJ, Schiff M, Pricop L, Shen S, Wang Z, Gaillez C. Secukinumab Sustains Individual Clinical Responses over Time in Patients with Psoriatic Arthritis: 2-Year Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/secukinumab-sustains-individual-clinical-responses-over-time-in-patients-with-psoriatic-arthritis-2-year-results-from-a-phase-3-trial/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-sustains-individual-clinical-responses-over-time-in-patients-with-psoriatic-arthritis-2-year-results-from-a-phase-3-trial/