Session Information
Date: Sunday, November 5, 2017
Title: Rheumatoid Arthritis – Clinical Aspects Poster I: Treatment Patterns and Response
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
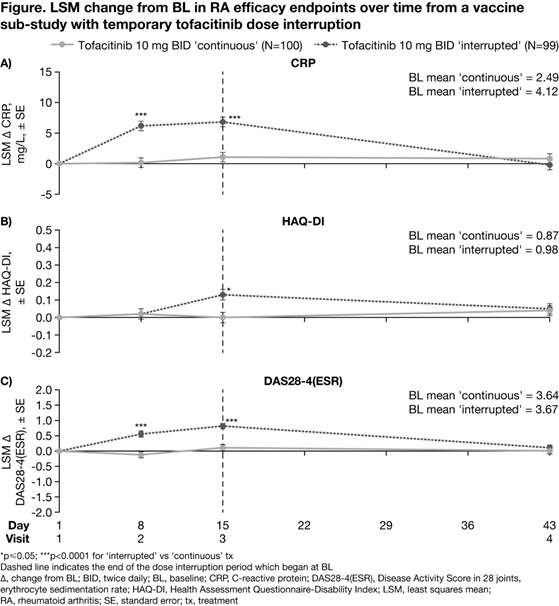
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). We assessed tofacitinib efficacy and safety after temporary discontinuation and reinitiation of therapy in patients (pts) with RA.
Methods: Data were from a randomized, parallel-group (grp), controlled, open‑label vaccine sub-study in pts with RA participating in a long-term extension study (NCT00413699). Pts were aged ≥18 years with active RA and had received tofacitinib 10 mg twice daily (BID) for ≥3 months. The sub-study included 2 treatment (tx) grps: ‘continuous tx’ (tofacitinib 10 mg BID ± methotrexate [MTX]) and ‘interrupted tx’ (tofacitinib withdrawn for 2 weeks post-randomization [Day 1–15; Visits 1–3], then tofacitinib 10 mg BID reinitiated ± MTX at Visit 3); randomization was stratified by MTX use. All pts received pneumococcal and influenza vaccines on Day 8 (Visit 2; vaccine titers reported previously1). Blood samples were taken on Days 8, 15 (Visit 3), and 43 (Visit 4). Efficacy endpoints included change from baseline (CFB) in CRP, HAQ‑DI, and DAS28-4(ESR) at each visit. A mixed-effects model with repeated measures was used to evaluate treatment effect at each visit. Efficacy analyses were exploratory, with no multiplicity adjustments. P<0.05 was considered as statistically significant.
Results: Of the 199 pts in this analysis (continuous, n=100; interrupted, n=99), 117 received concomitant MTX. At study baseline (BL) in the continuous and interrupted grps, respectively: 81.8/83.8% of total pts were white, 84.8/86.9% were female, and mean age was 55.0/53.9 years. CRP, HAQ-DI, and DAS28-4(ESR) BL values were generally similar between groups. At Day 8, mean CRP and DAS28-4(ESR) significantly increased from BL for pts receiving interrupted vs continuous tx; HAQ-DI values were similar between grps (Figure). At Day 15, mean CRP, HAQ-DI, and DAS28-4(ESR) significantly increased from BL for pts receiving interrupted vs continuous tx. After tofacitinib reinitiation for 28 days (Day 43), CFB in CRP, HAQ-DI, and DAS28-4(ESR) were similar between grps and approached BL levels. Adverse events (AEs) were experienced by 35.4% and 49.5% of pts receiving interrupted and continuous tx, respectively. The most frequent treatment-emergent AEs were bronchitis and upper respiratory tract infection (each AE: 6 pts) and vaccination-related immunisation reaction, myalgia, and rash (each AE: 5 pts). Serious AEs occurred in 3 pts (3%) in each grp. In total, 1 pt (1%) in the interrupted grp discontinued due to study drug-related AEs; no pts discontinued due to disease flare.
Conclusion: Efficacy of tofacitinib 10 mg BID can be re-established following a temporary (2 weeks) tx discontinuation in pts with RA. Pts receiving continuous tx maintained efficacy throughout the study. Further investigations are required.
Reference:
1. Winthrop KL et al. Ann Rheum Dis 2016; 75: 687-695.
To cite this abstract in AMA style:
Kaine J, Tesser J, DeMasi R, Takiya L, Wang L, Snyder M, Fan H, Wollenhaupt J. Re-Establishment of Efficacy of Tofacitinib, an Oral Janus Kinase Inhibitor, in Rheumatoid Arthritis Patients after Temporary Discontinuation [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/re-establishment-of-efficacy-of-tofacitinib-an-oral-janus-kinase-inhibitor-in-rheumatoid-arthritis-patients-after-temporary-discontinuation/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/re-establishment-of-efficacy-of-tofacitinib-an-oral-janus-kinase-inhibitor-in-rheumatoid-arthritis-patients-after-temporary-discontinuation/