Session Information
Date: Sunday, November 5, 2017
Title: Rheumatoid Arthritis – Clinical Aspects Poster I: Treatment Patterns and Response
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
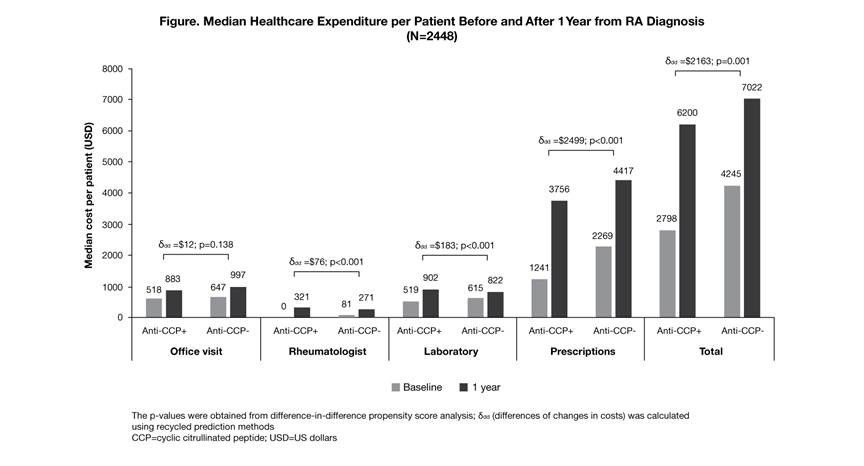
Background/Purpose: Anti-cyclic citrullinated peptide (anti-CCP) antibody positivity has been suggested as a strong predictor of joint erosion as well as a potential biomarker for guiding treatment decisions. There are limited data evaluating the economic burden of anti-CCP positivity. We investigated the association between anti-CCP positivity and healthcare expenditures in newly diagnosed patients (pts) with RA.
Methods: A retrospective cohort study was conducted in adult pts with RA in Kaiser Permanente Southern California (Jan 2007 to Dec 2014). Individuals were followed from their first RA diagnosis (index) for 12 months. Pts were required to have two International Classification of Diseases, Ninth Revision, RA diagnosis codes of 714.0–714.8, a DMARD prescription, and continuous eligibility for 12 months prior to and after index date. Pt demographics, anti-CCP status, co-morbidity and healthcare resource utilization during the study period were collected. Nationally recognized direct medical costs were assigned to healthcare utilizations to calculate healthcare costs in 2015 US dollars. Difference-in-difference (DID) propensity score analyses were conducted to determine the association between anti-CCP positivity and 12-month cost outcomes. A generalized linear regression model with recycled prediction methods was used to quantify the differences of changes in costs (ddd) between the anti-CCP-positive (anti-CCP+) and anti-CCP-negative (anti-CCP–) groups.
Results: A total of 2448 newly diagnosed pts with RA were identified with a mean (SD) age of 55.5 (14.3) years; 75.7% were female. At baseline, 65.8% of pts were anti-CCP+ and 34.2% were anti-CCP–, where anti-CCP+ pts had fewer co-morbidities (median Elixhauser co-morbidity scores: 3 vs 4; p<0.001) versus anti-CCP– pts. During follow-up, more anti-CCP+ pts received ≥1 biologic DMARD (22.6 vs 12.9%; p<0.001) and had more frequent rheumatologist visits (median number: 5 vs 4; p<0.001) versus anti-CCP– pts. During the 12-month follow-up, median (interquartile range) total healthcare expenditure for anti-CCP+ and anti-CCP– pts was $6200 ($3563–13,260) and $7022 ($3885–12,995), respectively. For the DID, when considering baseline costs, pt demographics and co-morbidities, anti-CCP positivity was significantly associated with higher prescription (ddd=$2499; p<0.001), laboratory testing (ddd=$183; p<0.001) and rheumatologist visit costs (ddd=$76; p<0.001). Total incremental cost associated with anti-CCP positivity was estimated as $2163 (p=0.001; Figure). No statistical differences were found in hospitalizations or emergency department-related costs.
Conclusion: In newly diagnosed pts with RA, the higher economic burden for anti-CCP+ pts was mainly driven by pharmacy costs. Future pt-reported outcomes and/or disease progression associated with the early economic burden may help guide treatment decisions.
To cite this abstract in AMA style:
An J, Bider Z, Kang J, Alemao E, Connolly S, Cheetham T. Economic Burden Associated with Anti-Cyclic Citrullinated Peptide Antibody Positivity in Patients Newly Diagnosed with RA [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/economic-burden-associated-with-anti-cyclic-citrullinated-peptide-antibody-positivity-in-patients-newly-diagnosed-with-ra/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/economic-burden-associated-with-anti-cyclic-citrullinated-peptide-antibody-positivity-in-patients-newly-diagnosed-with-ra/
