Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
– Background/Purpose: Neutrophil extracellular traps (NETs) are immunogenic, extracellular DNA structures that harness important autoantigens to be recognized by the adaptive immune system. NETs are thought to play a pivotal role in the pathogenesis of ANCA-associated vasculitis (AAV) and SLE. However it is still unclear how and if NETs can act as a common pathway in the pathophysiology of these clinically divergent autoimmune diseases. The aim of the present study is to characterize AAV- and SLE-induced NETs.
– Methods: The present study involved 88 AAV patients according to the Chapel Hill consensus definitions of 2012, 59 SLE patients according to the ACR criteria 1997 and 10 healthy controls. Healthy neutrophils were stimulated with 10% serum of AAV or SLE patients to induce NETs. Ex vivo NET induction by serum and IgG-depleted serum was measured by a novel, highly-sensitive NET quantification assay using 3D-confocal microscopy1. Qualitative characteristics of NETs were studied by immunofluorescence to detect NET-related auto-antigens. Additionally, the morphology and kinetics of AAV- and SLE-induced NETosis were visualized by live cell imaging and electron microscopy.
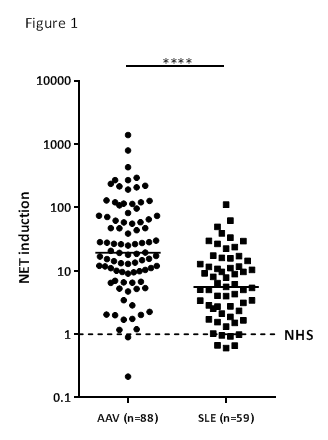
– Results: Ex vivo NET induction by AAV sera was 19.36 [9.161 – 73.08], (median [Q1 – Q3]) fold higher than sera of healthy controls (n=10) and also significantly higher than NET induction by SLE sera 5.56 [2.34 – 14.33] (Figure 1). Depletion of IgG from serum did not reduce NET induction in AAV, but it decreased NET induction significantly in SLE, indicating that different triggers mediate the induction of neutrophil extracellular traps in these autoimmune diseases. Additionally, the colocalisation of NET-related auto-antigens was different: citrullinated histon-3 (CitH3) was predominantly found on AAV-induced NETs, whereas high mobility group box protein-1 (HMGB-1) was exclusively found on SLE-induced NETs. Moreover, live cell imaging demonstrated that the kinetics of SLE-induced NETs peaked at 60 minutes, while AAV-induced NETs peaked at 4 hours (Figure 2). Intriguingly, SLE sera induced immediate clustering of neutrophils surrounding NETs whereas AAV sera induced NETs composed of long, thin DNA-fibres through lytic expulsion.
– Conclusion: We demonstrate intrinsically distinct features of AAV- and SLE-induced NETs, indicating that NET formation in AAV and SLE is based on different mechanisms. Future studies should be directed at unravelling how different NETs are involved in causing SLE- or AAV-associated glomerulonephritis.
1. T. Kraaij et al. – Autoimmunity Reviews 15 (2016) 577–584
To cite this abstract in AMA style:
van Dam L, Kraaij T, Kamerling S, Scherer HU, Pusey C, Rabelink T, van Kooten C, Teng O. Ex Vivo Induced Neutrophil Extracellular Traps Are Intrinsically Different in Anca-Associated Vasculitis and Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/ex-vivo-induced-neutrophil-extracellular-traps-are-intrinsically-different-in-anca-associated-vasculitis-and-systemic-lupus-erythematosus/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ex-vivo-induced-neutrophil-extracellular-traps-are-intrinsically-different-in-anca-associated-vasculitis-and-systemic-lupus-erythematosus/