Session Information
Title: Systemic Sclerosis, Fibrosing Syndromes and Raynaud’s – Pathogenesis, Animal Models and Genetics
Session Type: Abstract Submissions (ACR)
Background/Purpose: In scleroderma (SSc), lung fibrosis is linked to epithelial damage and dysregulated repair mechanisms. Resident lung fibroblasts may affect multiple cell types including epithelium, endothelium, smooth muscle cells and fibrocytes. We have used two complementary transgenic mouse strains with altered TGFβ signalling to better understand the regulatory role of resident lung fibroblasts in defining susceptibility to fibrosis.
Methods: The TβRIIΔk-fib mouse model of SSc, in which TGFβ signalling is upregulated in fibroblasts, is susceptible to fibrotic lung injury whereas the TβRII-null-fib strain, in which TβRII is conditionally knocked out in fibroblasts, is resistant to bleomycin-induced lung fibrosis. We have used an illumina® microarray platform to profile lung or skin fibroblasts from these two strains and identified a cohort of genes that determine susceptibility or resistance to experimental lung fibrosis, comparing to a control group using whole lung from TβRIIΔk-fib animals and wildtype littermates (n=3) on the same microarray platform. Technical validation of data and additional quantitation of gene expression was performed using quantitative RT-PCR assays with replicate samples.
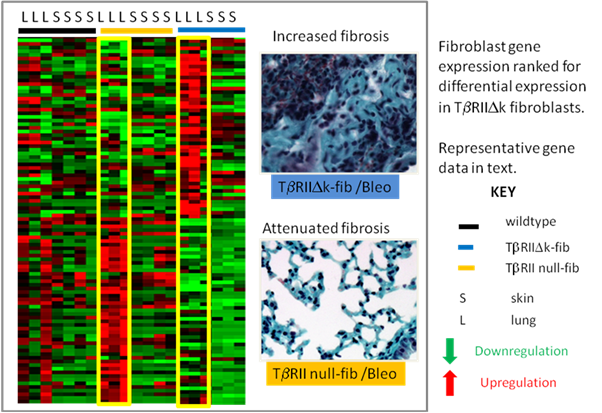
Results: The TβRIIΔk-fib lung fibroblast gene expression signature includes key genes that are implicated as pathogenic drivers of fibrosis and inflammation and potential biomarkers in SSc. Conversely, many of these genes are downregulated in TβRII-null-fib mice (figure 1), including BMP4 (fold reduction in TβRII-null-fib 31.8, p<0.02; fold upregulation in TβRIIΔk-fib compared with WT 2.01, p<0.6); elastin (TβRII-null-fib 17.8, p<0.14; TbRIIΔk-fib 1.86, p<0.09); CCL2 (TβRII-null-fib 56.8, p<0.09; TβRIIΔk-fib 1.72, p<0.03)and MMP13 (TβRII-null-fib 13.2, p<0.08; TβRIIΔk-fib 3.6, p<0.4). CTGF (CCN2) was strongly upregulated in TβRIIΔk-fib lung fibroblasts, but showed less downregulation than other genes in the TβRII-null-fib, probably reflecting multiple pathways of activation. No signature of overexpression was present in the whole lung analysis suggesting that fibroblast-specific differences in gene expression determine altered fibrotic response.
Conclusion: These data define a cohort of genes differentially expressed in fibroblasts that associate strongly with susceptibility or resistance to experimental lung fibrosis. These transcripts include many that are important in tissue repair and that have previously been shown to be over expressed in SSc skin samples. They suggest that resident fibroblast gene expression signature may govern fibrosis in lung and skin.
Disclosure:
E. Derrett-Smith,
None;
R. Hoyles,
None;
K. Khan,
None;
D. J. Abraham,
None;
C. P. Denton,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/resident-lung-fibroblast-gene-expression-signatures-predict-susceptibility-or-resistance-to-experimental-lung-fibrosis/