Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Cartilage lesions due to traumatic or pathological conditions slowly grow over the time and may lead to osteoarthritis (OA). As a prospective treatment for such lesions, it has been shown that human-derived induced pluripotent stem cells (iPSCs) can be 3D bioprinted and directed to form cartilage-like tissue (Nguyen et al. 2017). The advantages of using an established iPSC line are unlimited cell source with regeneration capacity and chondrogenic differentiation potential. The aim of this study was to improve the generation of cartilage-like tissue when 3D bioprinting of iPSCs by using molecularly modified nanocelullose/alginate bioink to resemble natural environment found in the tissue.
Methods: In this study the chondrocyte-derived iPSc line “A2B” was used (Borestrom et al. 2014). These cells were bioprinted in combination with a modified bioink composed by nanocellulose and alginate. One week after bioprinting, the constructs were cultured in chondrogenic medium in order to stimulate cell differentiation towards chondrocytes. Cell number and viability was studied. Histological analyses of the 3D printed constructs were performed. Furthermore, expression of pluripotency and chondrogenic specific genes was assessed by Taqman qPCR before and after differentiation.
Results: High viability of the iPSCs inside the constructs was found after bioprinting. 3D printed constructs were positively stained for alcian blue van gieson staining, showing proteoglycans presence inside the prints. Molecular analyses showed high relative expression levels of the pluripotency-related gene Oct4 before initiating the differentiation protocol. Cells inside the constructs express chondrogenic specific genes, such as collagen type 2 and Sox9 after 6 weeks of differentiation. Moreover, 3D printed constructs showed cartilage-resembles (Figure 1).
Conclusion : Modified Nanocellulose/alginate bioink allowed 3D printing of the iPSCs and the in vitro generation of cartilage-like tissue. This approach could be used in the future to model OA disease or to perform screenings of different therapeutic compounds.
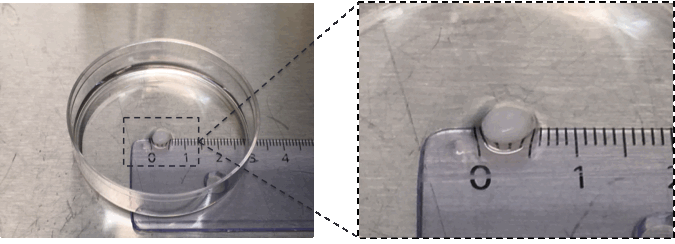 Figure 1. Cartilage mimic by 3D bioprinting iPSCs differentiated for 6 weeks in modified bio ink.
Figure 1. Cartilage mimic by 3D bioprinting iPSCs differentiated for 6 weeks in modified bio ink.
To cite this abstract in AMA style:
Castro-Viñuelas R, Forsman A, Karabulut E, Romberg E, Brantsing C, Brittberg M, Lindahl A, Gatenholm P, Simonsson S. Cartilage-like Tissue Generation By 3D-Bioprinting of Induced Pluripotent Stem Cells [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/cartilage-like-tissue-generation-by-3d-bioprinting-of-induced-pluripotent-stem-cells/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cartilage-like-tissue-generation-by-3d-bioprinting-of-induced-pluripotent-stem-cells/
