Session Information
Date: Wednesday, November 16, 2016
Title: Spondylarthropathies Psoriatic Arthritis – Pathogenesis, Etiology II
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: The pathogenesis of Axial Spondyloarthritis (AxSpA) is not well understood. A rare 9 base-pair deletion of SEC16A was recently identified to be strongly associated with AxSpA in a multiplex family. SEC16A plays an important role in endoplasmic reticulum (ER)-to-Golgi transport and the assembly of cargo carrier vesicles. We investigated if variations in SEC16A could affect the ER-Golgi transport of HLA-B27 and influence AxSpA pathogenesis.
Methods: EBV transformed B cells were isolated from 14 HLA-B27 positive members of the multiplex family. Out of 9 members with the SEC16A 9 bp deletion, 8 have been diagnosed with AxSpA and one 16-year-old was healthy. Five healthy family members had the wild-type (WT) SEC16A. C1R-B27 cells (B-lymphoblastoid cells with stable HLA-B27 expression) were used to replicate findings by knocking out (KO) SEC16A using CRISPR. Fluorescent tagged monoclonal antibodies against SEC16A and SEC31A (identifies ER-to-Golgi transport vesicle COPII) were used. The formation of ER-to-Golgi transport vesicles and the organization of ER exit sites (ERES) were studied using confocal microscopy. The vesicular stomatitis virus glycoprotein (VSVG-GFP) was used to track ER-to-Golgi transport. In addition, Fluorescence Recovery After Photobleaching (FRAP) was performed to study the dynamics of ER-to-Golgi transport vesicles. Changes in surface and intracellular HLA-B27 and free heavy chain (FHC) expression were tested by flow cytometry. Activation of unfolded protein response (UPR) was measured using PCR and western blot for BiP, CHOP and ATF-6.
Results: EBV-B cells with the SEC16A mutation had a significantly (P<0.001) lower number of secretory vesicles at ERES (co-localization of SEC16A and SEC31A in Figure 1A) and impaired ER-to-Golgi transport (decreased VSVG-GFP trafficking). By FRAP, there was poor recruitment of SEC31A to ERES. There was significantly lower surface expression of intact HLA-B27 and FHC (P<0.01) and accumulation of both intracellularly (P<0.01). All findings were replicated in C1R-B27 cells after 90 % SEC16A suppression. UPR was significantly elevated due to the insufficient ER-Golgi transport in the SEC16A knock out C1R-B27 cells (Figure 1B).
Conclusion: Thus SEC16A is an important factor that can influence HLA-B27 trafficking, surface and intracellular expression and UPR. Abnormal SEC16A-HLA-B27 interaction may play an important role in the pathogenesis of AxSpA. Figure 1. A 9-bp deletion of SEC16A (MUT) decreases COPII vesicle formation compared to wild Type SEC16A (WT) (A). SEC16A knockout (90%) leads to increased unfolded protein response (B). 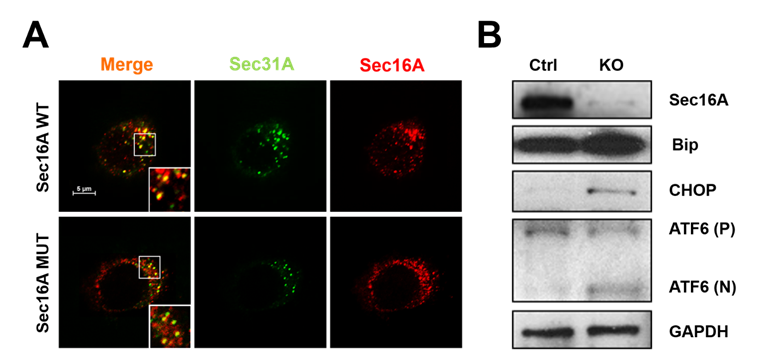
To cite this abstract in AMA style:
Zeng F, Zhang Z, Ranganathan V, Orielly D, Rahman P, Haroon N. SEC16A and Intracellular Trafficking Abnormalities in Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/sec16a-and-intracellular-trafficking-abnormalities-in-axial-spondyloarthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sec16a-and-intracellular-trafficking-abnormalities-in-axial-spondyloarthritis/
