Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Considerable efforts are currently being directed at developing robust and efficient trial designs to study the efficacy of disease modifying drugs (DMOADs) for osteoarthritis (OA). As part of this effort, an understanding of patients’ preferences is needed.
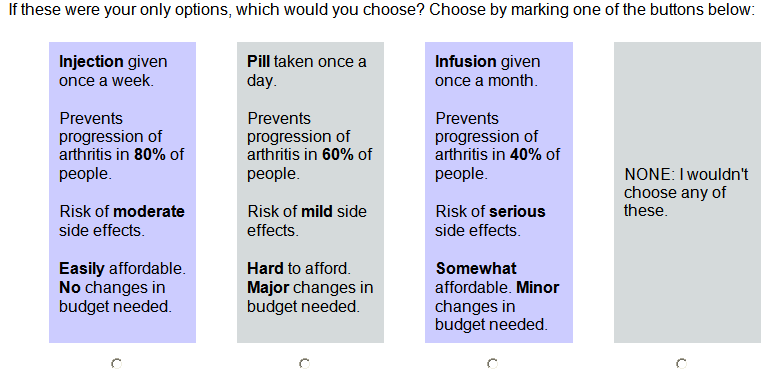
Methods: We administered a conjoint analysis survey to a convenience sample of 304 patients attending outpatient clinics. The survey was composed of 4 attributes each having 3 levels: (1) administration (pill, injection (SC), infusion (IV)), (2) benefit (prevents progression in 40%, 60%, or 80%), (3) risk (mild: < 1 week and reversible, moderate: (1-2 weeks and requires treatment, serious: requires hospitalization), and (4) cost (easy, somewhat, hard to afford). All subjects were provided with a detailed description of each attribute before performing the survey. The survey included 12 choice tasks each with 3 medications and a “None” option (Figure). We performed Latent Class Segmentation analysis and simulations to estimate preferences for 4 options: Best Case (pill, prevents progression in 80%, mild side effects, easy to afford), Worst Case (IV, prevents progression in 40%, serious side effects, hard to afford), SC (SC, prevents progression in 40%, moderate risk, somewhat hard to afford), IV (same as previous except IV).
Results: 304 subjects participated; median (range) age=57 (34-89); 55% female; 69% Caucasian, 45% employed; 37% college graduates; 29% reported knee pain often or very often; 30% reported physician diagnosed knee arthritis. Segmentation analysis revealed 4 distinct groups of patients. The relative importances of the attributes are presented in Table 1. Treatment preferences are described in Table 2. Group 1 (5%) do not want to perform injections and only consider DMOADs under the Best Case scenario; Group 2 (19.4%) are most influenced by risk and fewer prefer DMOADs under more realistic scenarios; Group 3 (16.4%) consistently reject DMOADs, and Group 4 (59.2%) strongly prefer DMOADs regardless of risk or cost.
Conclusion: Almost 60% of patients surveyed are willing to accept substantial risk in order to prevent progression of OA. These findings should help inform future drug development.
Table 1. Relative Importances of Each Attribute
|
Attribute |
Group 1 (N=15) |
Group 2 (N=59) |
Group 3 (N=50) |
Group 4 (N=180) |
|
1. Administration |
58.5 |
11.5 |
11.8 |
2.9 |
|
2. Benefit |
12.1 |
22.9 |
27.5 |
50.5 |
|
3. Risk |
14.6 |
35.7 |
28.5 |
24.6 |
|
4. Cost |
14.8 |
29.8 |
32.2 |
22.1 |
Table 2. Percent Preferring Each Hypothetical Treatment Option
|
Scenario |
Group 1 (N=15) |
Group 2 (N=59) |
Group 3 (N=50) |
Group 4 (N=180) |
|
Best case |
99.4 |
98.2 |
18.4 |
89.8 |
|
Worst case |
0.7 |
4.4 |
7.6 |
85.4 |
|
SC |
5.3 |
67.2 |
12.4 |
86.3 |
|
IV |
20.2 |
54.9 |
11.1 |
86.1 |
Disclosure:
L. Fraenkel,
None;
C. Cunningham,
None;
G. A. Hawker,
None;
L. Suter,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-patient-preferences-to-inform-the-development-of-disease-modifying-drugs-for-osteoarthritis/