Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with psoriatic arthritis (PsA) or psoriasis have an increased risk of cardiovascular morbidity and mortality. The short-term impact of biologic therapies on cardiovascular events is still debated. The objective of this meta-analysis was to investigate the association between biologic therapies and major adverse cardiac events (MACEs) or congestive heart failure (CHF) in PsA or psoriasis patients.
Methods: Study screening was performed using MEDLINE and Cochrane, from database inception to April 2016, including randomized controlled trials (RCTs) of anti-IL12/23 (briakinumab, guselkumab and ustekinumab), anti-IL17 (brodalumab, ixekizumab and secukinumab) or anti-TNF (adalimumab, certolizumab, etanercept, golimumab and infliximab) agents for the treatment of PsA or psoriasis. Two investigators independently performed data extraction of MACEs and CHF events reported during the placebo-controlled phase of biologic therapies. These outcomes were presented as risk differences with their 95% confidence interval for each selected study. Meta-analyses were carried out independently in PsA or psoriasis, using the inverse variance method. Heterogeneity was tested with the Cochran’s Q-test and evaluated by the I2 statistic. We used RevMan 5.3 for the meta-analysis calculations. P-values less than 0.05 were considered statistically significant.
Results: On 415 studies initially screened, 56 studies were finally selected: 16 in PsA (1339.54 patient-years [P-Y]) and 40 in psoriasis (5128.96 P-Y). Compared with placebo, no significant difference was observed in MACEs in patients receiving anti-IL12/23, anti-IL17 or anti-TNF agents, neither in PsA nor in psoriasis. However, in psoriatic patients, 11 MACEs were observed in the anti-IL12/23 group (946 P-Y) compared to 0 in placebo group (493 P-Y), with 0.01 [-0.00 to 0.02] event / P-Y risk difference, which is not statistically significant (Figure). Similarly, no significant difference was observed in CHF incidence in patients receiving biologic agents, neither in PsA nor in psoriasis.
Conclusion: Even if there was no statistically significant difference in the incidence of MACEs or CHF in patients receiving biologic therapies during the placebo-controlled phase of RCTs, physicians must remain aware of the risk of MACEs or CHF when initiating a biologic therapy in PsA or psoriasis. 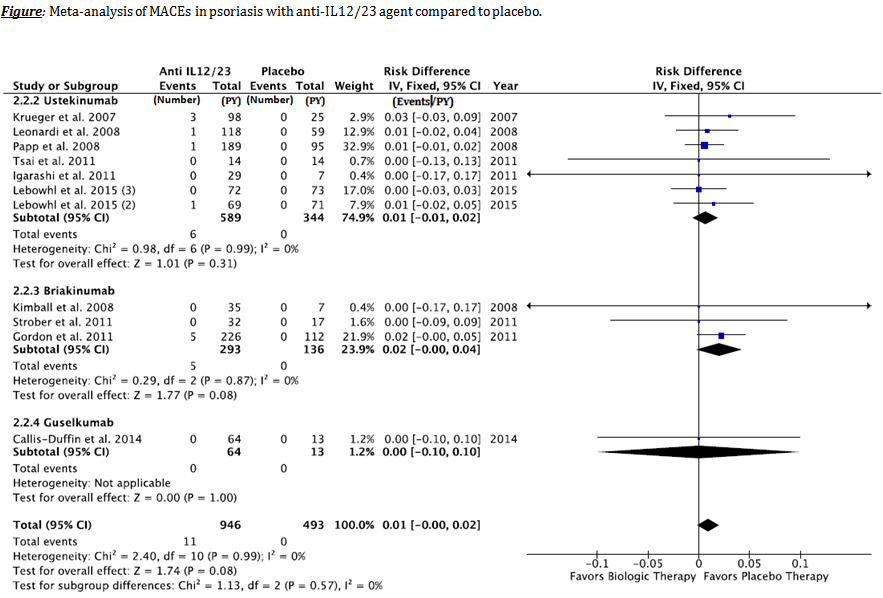
To cite this abstract in AMA style:
Champs B. Association Between Biologic Therapies and Major Adverse Cardiac Events or Cardiac Heart Failure in Psoriatic Arthritis or Psoriasis: A Meta-Analysis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/association-between-biologic-therapies-and-major-adverse-cardiac-events-or-cardiac-heart-failure-in-psoriatic-arthritis-or-psoriasis-a-meta-analysis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-biologic-therapies-and-major-adverse-cardiac-events-or-cardiac-heart-failure-in-psoriatic-arthritis-or-psoriasis-a-meta-analysis/
