Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The majority of patients (pts) with psoriatic arthritis (PsA) experience psoriatic skin manifestations, which add to the already high burden of disease. The RAPID-PsA trial (NCT01087788) of certolizumab pegol (CZP) in PsA has shown CZP to be efficacious in improving skin manifestations over 96 weeks (wks) of treatment.1 Here, we report long-term efficacy data for skin manifestations over 4 years of the RAPID-PsA trial.
Methods: The phase 3 RAPID-PsA trial was double-blind and placebo-controlled to Wk 24, dose-blind to Wk 48 and open-label (OL) to Wk 216. Pts had active PsA and had failed ≥1 DMARD; up to 40% of pts could have received 1 prior anti-TNF. Pts originally randomized to CZP (200 mg Q2W or 400 mg Q4W, following 400 mg loading dose at Wks 0, 2, 4) continued on their assigned dose in the OL period. Here we report outcomes assessing the skin manifestations of PsA in CZP-randomized pts with skin involvement at baseline (≥3% body surface area affected by psoriasis [BSA]). Data are shown as observed case (OC) and with imputation: NRI for categorical measures and LOCF for continuous measures.
Results: Of the 409 pts randomized, 273 received CZP from Wk 0, 166 of whom had baseline skin involvement (≥3% BSA). At baseline, these patients had an average of 24.2% BSA and a mean PASI score of 12.0. Severe skin involvement (≥3% BSA and PASI ≥10) was seen in 71 pts at baseline, with an average of 38.7% BSA affected and a mean PASI score of 22.3 amongst these pts. In pts completing the study, early improvements in PASI responses observed to Wk 241 were sustained to Wk 216 following treatment with either dose regimen (Table). Even when conservative imputation methods (NRI) were used, PASI responses were largely maintained, with an increased proportion of pts achieving the most stringent outcome, PASI100 (NRI; Wk 24: 22.3%, Wk 216: 28.3%). Similar sustained improvements were observed in both mean BSA and mean PASI score (Table). At Wk 48, 77.5% of pts with severe skin involvement at baseline (PASI ≥10) achieved a PASI75 response, compared with 54.7% of pts with less severe baseline skin involvement (PASI <10) (NRI). The heightened response in the most severely affected pts remained evident at Wk 216 (PASI75 [NRI]: PASI ≥10: 59.2%, PASI <10: 46.3%).
Conclusion: Improvements in the skin manifestations of PsA, as seen at Wk 24,1 were maintained over 4 years of CZP treatment with both dose regimens, with additional improvements observed at Wk 216 in the proportion of pts achieving the most stringent measure, the PASI100 response. The increased PASI75 response rate previously observed in pts with severe skin involvement relative to those with less severe skin manifestations was also maintained to Wk 216. References: 1. Mease P. Ann Rheum Dis 2014;73:48–55 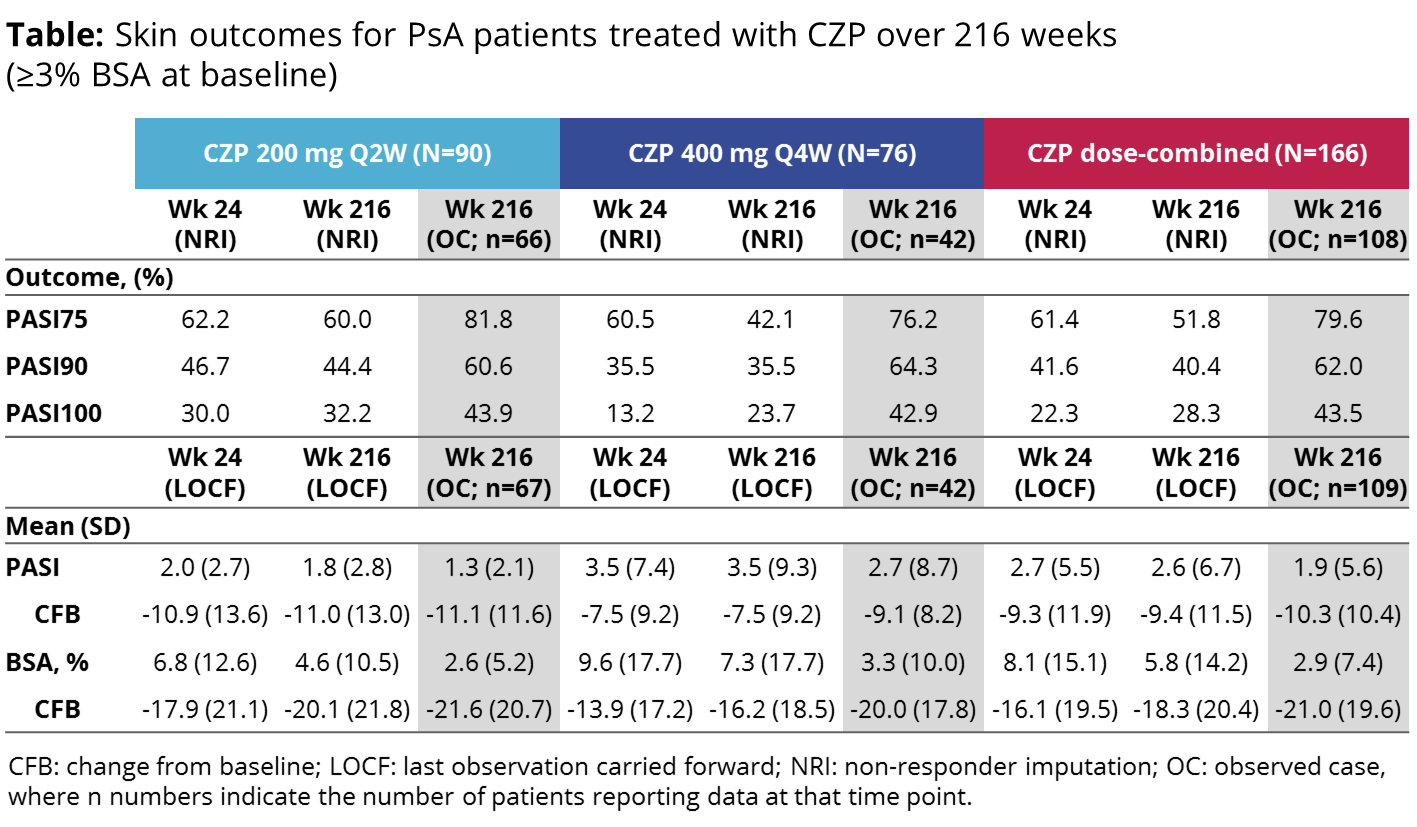
To cite this abstract in AMA style:
Khraishi M, Gottlieb AB, Hoepken B, Peterson L, Mease PJ. The Effect of Certolizumab Pegol on Skin Manifestations of Psoriatic Arthritis over 4 Years of Treatment [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-certolizumab-pegol-on-skin-manifestations-of-psoriatic-arthritis-over-4-years-of-treatment/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-certolizumab-pegol-on-skin-manifestations-of-psoriatic-arthritis-over-4-years-of-treatment/
