Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tofacitinib is a novel oral Janus kinase inhibitor being investigated as a targeted immunomodulator and disease-modifying therapy for RA. In the Phase 3 ORAL Scan study [NCT00847613], progression in radiographic scores (mean change from baseline [BL] in modified Total Sharp Scores [mTSS] at Month 6) was a primary analysis using an Analysis of Covariance (ANCOVA). ANCOVA demonstrated statistically significant inhibition in structural damage progression for tofacitinib 10 mg but not 5 mg twice daily (BID) doses, compared with placebo (PBO).1 There has been a general trend towards less PBO progression in recent radiographic studies;2 the PBO period is generally shorter (≤3 months) and most patients receiving PBO are rescued. It is therefore important to confirm that efficacy detected in a primary analysis is robust. Rank analysis of ORAL Scan data demonstrated borderline evidence of inhibition by both doses. Rank analyses are used to show results are not driven primarily through extreme values, as ranking down weights extreme values relative to ANCOVA, but analyses of ranks do not yield intuitively interpretable values representing the magnitude of damage inhibition. A conceptual bridge between rank analyses and ANCOVA is the trimmed-analysis approach, where extreme values are systematically removed from the data set.
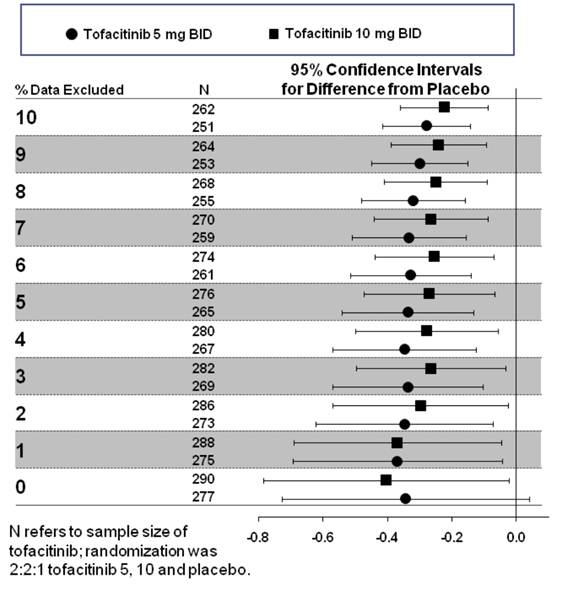
Methods: In this analysis, mTSS data were trimmed in 1% increments up to 10%, eg 2% trimming deletes observations <1st percentile and >99th percentile. In the ORAL Scan study this resulted in approximately 2-3 observations symmetrically removed per group per 1% trimming. ANCOVA was then applied to each resulting trimmed set (0% was the primary analysis).
Results: At 1% trimming, nominal statistical significance was achieved for both tofacitinib doses (CI less than 0) and was maintained with further trimming (Figure). As expected, the mean difference from PBO diminished slightly with trimming, but was more than compensated for by reductions in variability.
Conclusion: In ORAL Scan, significance of inhibition of structural damage was demonstrated for both tofacitinib doses after just 1% trimming, indicating that significance in the primary analysis was not dependent on extreme data. Trimmed analyses give improved insight into the influence of extreme values and should be considered as one of the sensitivity analyses of choice for structural data.
References: 1. van der Heijde et al. Arthritis Rheum 2011; 63: S1017-18. 2. Rahman et al. Ann Rheum Dis. 2011;70:1631-40.
Figure.
Trimmed data = % Data Excluded
Disclosure:
R. B. M. Landewé,
Pfizer Inc., Abbott, Janssen, Merck,
2,
Abbott, Amgen, Astra, Bristol-Myers Squibb, Centocor, GlaxoSmithKline, Janssen, Pfizer, UCB, Vertex,
5;
D. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocon, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth ,
5,
Imaging Rheumatology,
4;
C. Connell,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
J. Bradley,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
D. Gruben,
Pfizer Inc.,
3,
Pfizer Inc.,
1;
M. Brown,
Pfizer Inc.,
1,
Pfizer Inc.,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trimmed-analyses-a-new-approach-to-the-analysis-of-sharp-score-data-in-the-assessment-of-progression-in-patients-with-rheumatoid-arthritis/