Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Patients with systemic lupus (SLE) often have increased type I interferon levels (IFN-I) and activation of IFN-inducible genes (IFN signature). The mitochondrial adaptor protein MAVS (also known as IPS1, VISA or CARDIF) is a key intermediary in the RIG-I pathway, where viral RNA triggers a conformational change in RIG-I, leading to MAVS activation and then downstream activation of IKK and TBK1, with subsequent IFN production driven by IRF-3/7 (IRF3 for IFN-beta; IRF7 for IFN-alpha) and NFkB activation and translocation. Using in vitro methods, it has been observed that MAVS may form large prion-like aggregates that might stimulate IFN-I activation in a potent and prolonged fashion (Hou et. al., Cell 146:448, 2011). We wondered if such aggregates might be detectable ex vivo in SLE patients, and whether they might play a role in the sustained increased production of IFN-I.
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from 17 patients fulfilling ACR criteria for SLE, and from 9 controls. Thirty million PBMCs were lysed and supernatants loaded onto semi-denaturing 1.5% vertical agarose gels. After electrophoresis, the proteins were transferred to membranes for immunoblotting with anti-MAVS antibody or anti-beta-actin.
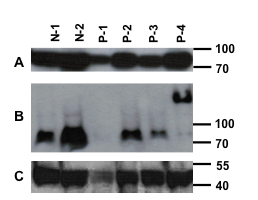
Results: Four of 17 SLE patients showed clear MAVS aggregation, with essentially all of their MAVS protein in a high molecular weight aggregated form. None of 9 controls had abnormal MAVS. Three of the four aggregation-positive SLE patients had nephritis and the fourth had lung involvement. SLEDAI scores of MAVS-aggregate positive SLE patients did not differ from patients with normal molecular weight MAVS. Patient 4 (P-4) shows the aggregated MAVS phenotype in the western blot below (Panel B, P-4). Denatured MAVS immunoblotting is shown in panel A and actin immunoblotting in panel C. N-1 and N-2 are normal controls. P-1 has less protein loaded and no MAVS band is discernible.
Conclusion: This is the first report of aggregated MAVS in human cells. The significance of this abnormality needs further investigation, it is possible that prolonged and increased IFN-I production could result from such MAVS aggregation, and that the poorly degradable prion-like protein could signal IFN-I production for prolonged periods.
Disclosure:
P. L. Cohen,
None;
W. H. Shao,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spontaneous-aggregation-of-the-anti-viral-mavs-protein-in-certain-systemic-lupus-erythematosus-patients-may-explain-excessive-type-i-interferon-production/