Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Corticosteroids (CSs) are widely used in Systemic Lupus Erythematosus (SLE) patients, but have side-effects when used for prolonged periods of time. Our aim was to obtain a better understanding of the relationship between exposures to CSs and adverse events (AEs) in a large trial.Methods:
We used data from the BLISS-76 trial, phase 3 randomized, placebo-controlled study of belimumab1. Reported AEs were grouped according to medical relevance. The frequencies of these groups were compared between the lowest versus the highest tertile of cumulative CS doses. Additionally, we studied the relationship between AEs and patients with and without CSs at baseline.Results:
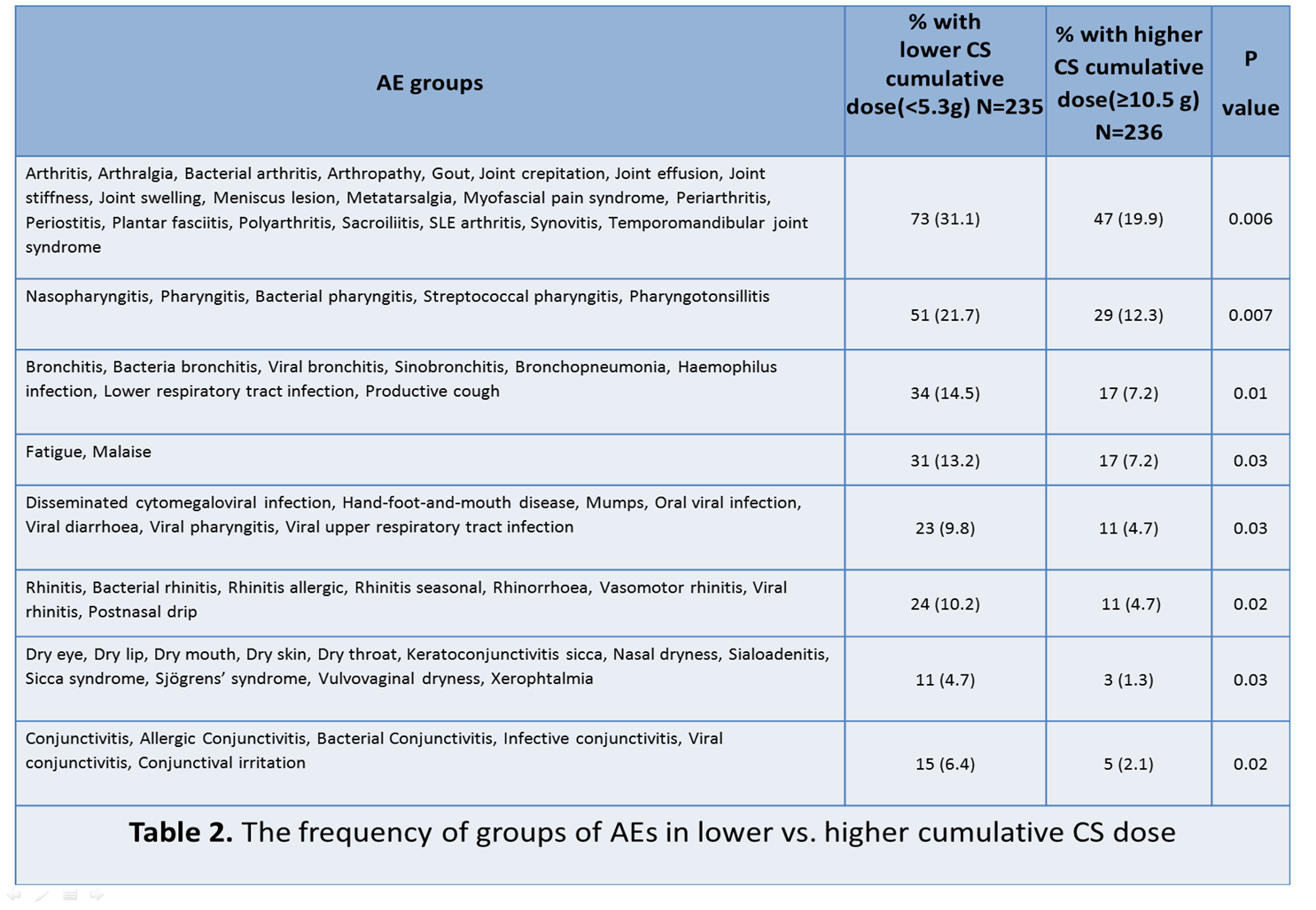
991 AEs were reported in 819 patients of the trial. Of 714 patients (86%) treated at least once with CSs prior/during the trial, 235 were in the lowest tertile of cumulative CS dose(<5.3g) and 236 in the highest (≥10.5g) tertile. The following AEs: viral upper respiratory tract infection, viral gastroenteritis, allergic rhinitis, sinusitis, arthralgia, dental caries and bronchitis were more frequent in the lowest as compared to the highest tertile of CS doses. Only tachycardia and proteinuria were significantly more frequent in the highest tertile (Table1). Eight groups of AEs were more frequent in the lowest tertile as compared to the highest (Table2). At baseline, 76% of patients were treated with CSs. The frequencies of 4 AEs (anaemia, pyrexia, oral herpes and malaise) were significantly higher compared to those not treated with CSs. Conversely, the frequencies of several AEs was higher in patients without CSs at baseline (Table 3), most notably; asthma, infusion related reaction, nausea, seasonal allergy, sinusitis and viral upper respiratory tract infection.
Conclusion: Our study demonstrates the association of CSs with CS-specific AEs in a large RCT and highlights the feasibility of post-hoc analysis of data from RCT to extract valuable safety signals. Contrary to expectations, there were also associations between lower cumulative CS dosage and a range of AEs. 

Disclosure: S. Emamikia, None; C. Gentline, None; M. Backheden, None; K. Chatzidionysiou, None; L. Arnaud, GSK, 5; R. F. van Vollenhoven, AbbVie, Amgen, BMS, GSK, Pfizer, Roche, UCB,Biotest,Crescendo,Janssen, Lilly, Merck, Vertex, 9.
To cite this abstract in AMA style:
Emamikia S, Gentline C, Backheden M, Chatzidionysiou K, Arnaud L, van Vollenhoven RF. Relationship Between Corticosteroids and Adverse Events in SLE –Data from the Clinical Trial Belimumab in Subjects with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/relationship-between-corticosteroids-and-adverse-events-in-sle-data-from-the-clinical-trial-belimumab-in-subjects-with-systemic-lupus-erythematosus/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationship-between-corticosteroids-and-adverse-events-in-sle-data-from-the-clinical-trial-belimumab-in-subjects-with-systemic-lupus-erythematosus/
