Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Anti-TNFs neutralize the TNF-mediated component of inflammatory diseases such as rheumatoid arthritis (RA) and Crohn’s disease (CD) to reduce disease activity. In this study, we aimed to identify the range of plasma certolizumab pegol (CZP) concentrations ([CZP]) associated with optimal improvement in disease activity for patients (pts) with RA and CD exposed to different dosing regimens.
Methods: Efficacy endpoints by [CZP] were assessed using two methods: 1) correlation of measured [CZP] with disease activity (RA); 2) population PK/PD modeling (RA and CD). Observed RA data were from RAPID1 and RAPID2. Data for PK/PD modeling were pooled across multiple placebo-controlled CZP studies in RA and CD. RA pts were treated with a loading dose (CZP 400 mg at Weeks [Wks] 0, 2, 4) followed by a maintenance dose (RA: 200 mg or 400 mg Q2W). CD modeling was performed for the loading dose (CZP 400 mg at Wks 0, 2, 4) during induction phase, and maintenance dose (400 mg Q4W). [CZP] was measured using an ELISA validated in line with FDA/EMA regulatory requirements for bioanalytical methods. In RA, [CZP] was correlated with change from baseline in DAS28(CRP) (ΔDAS) and clinical disease activity index at Wks 12 and 24. In CD, [CZP] was correlated with Crohn’s disease activity index (CDAI) remission (≤150 points), CRP response (≤5 mg/L), fecal calprotectin (FC) response (≤250 μg/g) and composite outcome (CDAI ≤150 and FC ≤250 µg/g) at Wks 6 and 26.
Results: In RAPID1/RAPID2 (n=1479 RA pts; data pooled for CZP 200 mg Q2W and 400 mg Q2W), the interquartile range (IQR) of measured [CZP] was 20–50 μg/mL, associated with ΔDAS ≥2.0 at Wk 12, and ΔDAS ≥2.4 at Wk 24 (Figure A). A similar [CZP] range was associated with improvement in clinical disease activity index (not shown). PK/PD modeling in RA (n=2621 pts) confirmed the [CZP] range observed for the CZP 200 mg Q2W and 400 mg Q2W regimens, with [CZP] ≥24 μg/mL associated with ΔDAS ≥2 at Wks 12 and 24. For the 400 mg Q4W regimen, similar ΔDAS improvements were predicted for [CZP] ≥15 μg/mL. In CD (n=2157 pts), receiver operating curve analysis showed a loading dose [CZP] of 36 μg/mL (Wk 6) and a maintenance [CZP] of 15 μg/mL (Wk 12) associated with response for the various outcomes analyzed (Figure B). In both RA and CD, predicted [CZP] thresholds associated with response were consistent with the IQRs of measured [CZP] (Figure C).
Conclusion: Based on these ELISA data, in pts with RA treated with the CZP 200 mg Q2W or 400 mg Q2W regimens, ΔDAS increased with [CZP] up to 20 μg/mL; the majority of pts had [CZP] ≥20 μg/mL, which was associated with a plateau effect in ΔDAS from Wk 12, averaging ~2.0 at Wk 12 and ~3.0 at Wk 24. PK/PD modeling predicted a lower [CZP] range for the 400 mg Q4W regimen, with similar efficacy. In pts with CD, the loading dose [CZP] range and association with efficacy were in line with RA results; maintenance [CZP] ranged 4–28 μg/mL. 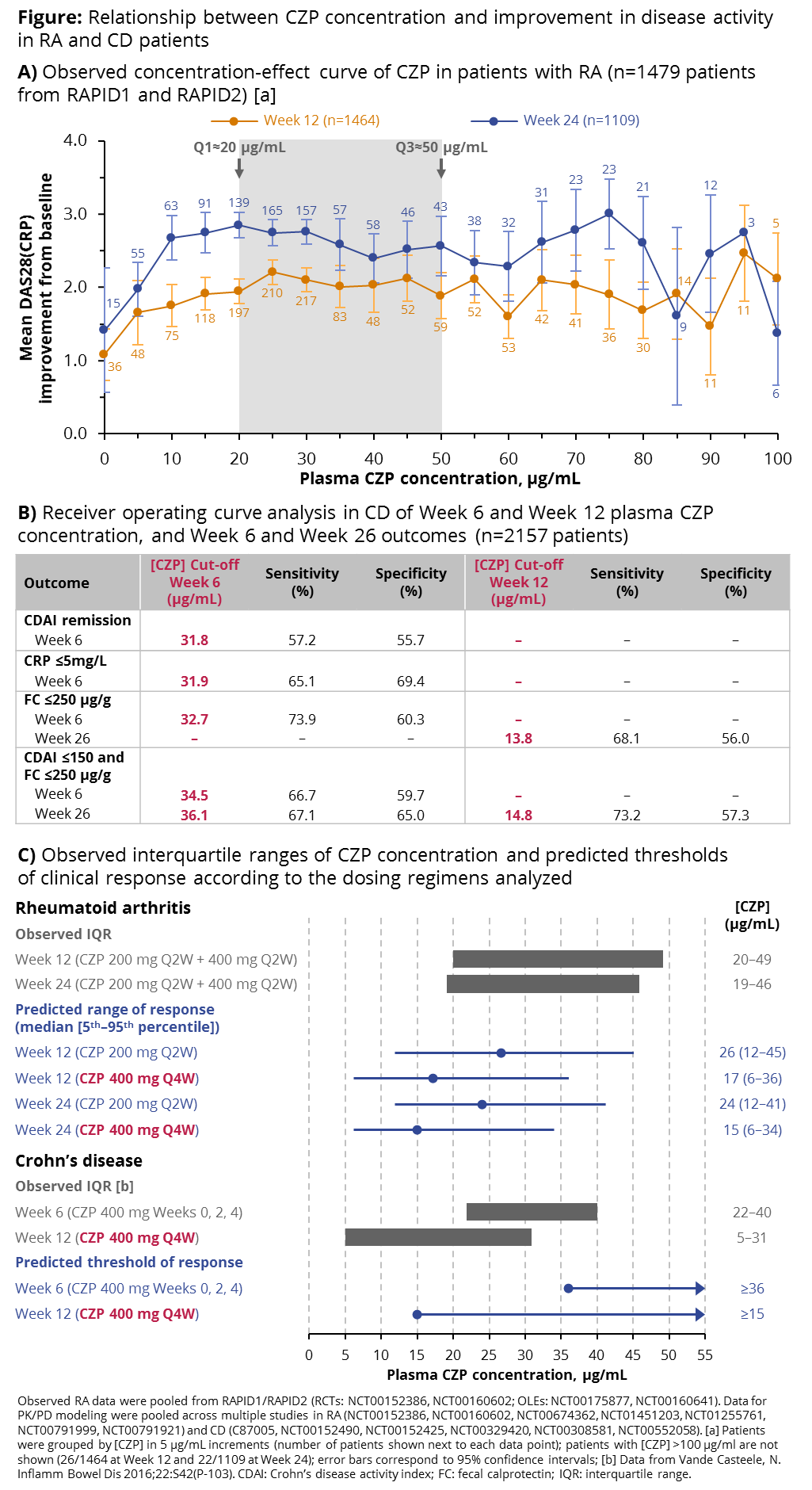
To cite this abstract in AMA style:
Wolbink G, Goupille P, Sandborn W, Marotte H, Mulleman D, Ternant D, Paul S, de Longueville M, Vande Casteele N, Zamacona M, O'Brien C, Kvien TK, Kavanaugh AF. Association Between Plasma Certolizumab Pegol Concentration and Improvement in Disease Activity in Rheumatoid Arthritis and Crohn’s Disease [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/association-between-plasma-certolizumab-pegol-concentration-and-improvement-in-disease-activity-in-rheumatoid-arthritis-and-crohns-disease/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-plasma-certolizumab-pegol-concentration-and-improvement-in-disease-activity-in-rheumatoid-arthritis-and-crohns-disease/
