Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Human Etiology and Pathogenesis - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: One unique feature of RA is the presence of ACPA. PTPN22 is a phosphatase that also acts to suppress citrullination independently of its phosphatase activity by inhibiting the function of peptidyl arginine deiminases (PADs). A C-to-T single nucleotide polymorphism, converting an arginine (R620) of PTPN22 to a tryptophan (W620), renders PTPN22 unable to suppress PADs and is associated with higher risk of RA and hypercitrullination in PBMC. Th cells of RA patients are prone to differentiation into Th17 cells and display hypoglycolysis, hyperproliferation, and excessive reductive stress due to abnormal expression of PFKFB3, ATM, and G6PD. We postulate that these abnormal features and hypercitrullination predate the onset of clinical symptoms of RA and can be attributed to attenuated phosphatase and/or non-phosphatase activities of PTPN22.
Methods: PBMC were collected from healthy at-risk individuals (ARIs), including RA first-degree relatives and/or ACPA+ individuals, and healthy ACPA- control individuals. The PBMC were stimulated with anti-CD3. Intracellular staining, western blotting, and real time PCR were used to quantify the level of citrullinated proteins and the expression of various genes. The levels of cytokines and lactate in supernatant were measured with ELISA and a colorimetric assay. PBMC were also transfected with plasmid vectors expressing PTPN22 or PADs to examine the impact of these proteins on the phenotype of PBMC.
Results: Regardless of the ACPA status, a high level of citrullinated histone H3 (cit-H3) was detected in ARIs but not in the control populations. T cells were the major source of cit-H3 in ARI PBMC. Despite a normal level of G6PD, ARI PBMC expressed more IL-2 and IL-17, but less IL-4, ATM and PFKFB3, and were hypoglycolytic. These abnormal features and hypercitrullination correlated with impaired anti-CD3-mediated induction of PTPN22 and were rectified by exogenous PTPN22. A phosphatase-dead PTPN22 was still able to normalize the level of cit-H3, IL-4 and IL-17 but not IL-2, ATM, or PFKFB3. In contrast, W620-PTPN22 normalized the expression of IL-2, ATM, and PFKFB3, but not IL-4 or IL-17. Furthermore, forced expression of PAD2 or PAD4 in control PBMC induced hypercitrullination and reduced the expression of IL-4, whereas only PAD2 was able to enhance the expression of IL-17.
Conclusion: Our data depict a molecular timeline of preclinical RA (Figure 1): impaired induction of PTPN22 leading to attenuated phosphatase activity and PADs-mediated hypercitrullination. The attenuated phosphatase activity results in aberrant expression of IL-2, ATM, and PFKFB3, whereas PAD2- or PAD4-mediated hypercitrullination inhibits the expression of Th2 cytokine. However, only PAD2-mediated hypercitrullination can augment the expression of Th17 cytokines. All of these events take place with or without the development of ACPA and abnormal expression of G6PD. 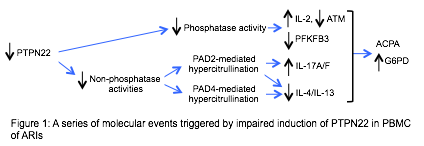
To cite this abstract in AMA style:
Chang HH, Dwivedi N, Sun B, Rao DA, Sparks JA, Kinslow J, Okamoto Y, Deane KD, Demoruelle MK, Norris JM, Karlson E, Holers VM, Ho IC. A Molecular Timeline for Preclinical RA Pathogenesis Defined By Dysregulated PTPN22, Hypercitrullination, and Aberrant Cytokine/Metabolic Profiles in PBMC of at-Risk Individuals [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/a-molecular-timeline-for-preclinical-ra-pathogenesis-defined-by-dysregulated-ptpn22-hypercitrullination-and-aberrant-cytokinemetabolic-profiles-in-pbmc-of-at-risk-individuals/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-molecular-timeline-for-preclinical-ra-pathogenesis-defined-by-dysregulated-ptpn22-hypercitrullination-and-aberrant-cytokinemetabolic-profiles-in-pbmc-of-at-risk-individuals/
