Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Clinical Aspects - Poster I: Clinical Characteristics/Presentation/Prognosis
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Adipose tissue macrophages (ATMs) are a potent source of inflammatory cytokines with profound effects on adipose tissue function and systemic inflammation, yet their potential role in rheumatoid arthritis (RA) pathobiology is unstudied.
Methods: Periumbilical subcutaneous adipose tissue was obtained from 36 RA patients and 22 non-RA controls frequency matched on demographics and body mass index (BMI). Individuals with diabetes were excluded. Samples were stained for the macrophage marker CD68 and the average proportion of ATMs relative to total nucleated cells was compared between groups. Using real time polymerase chain reaction (RT-PCR), gene expression profiles for selected inflammatory cytokines, chemokines, and macrophage markers were assessed and their correlations with RA disease characteristics were explored.
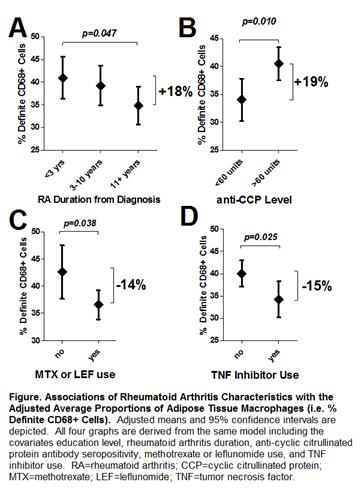
Results: The RA and control groups were well matched [mean age=54 years, 75% female, mean BMI=28.1]. Median RA duration was 6.3 years and 77% were seropositive for rheumatoid factor (RF) or anti-CCP antibodies. A total of 78% were treated with non-biologic DMARDs, 44% with biologics, and 25% with prednisone. The proportion of ATMs was 76% higher in RA vs. non-RA samples (37.7 vs. 21.3%, respectively; p<0.001). ATMs aggregated into crown-like structures (CLSs) around adipocytes were more than 1.5-fold higher in the RA group compared with controls (0.58 vs. 0.23 CLSs/high-power field, respectively; p=0.001). ATMs were significantly more abundant in early RA and in those seropositive for anti-CCP (Fig1A&B). Users of methotrexate, leflunomide, and TNF inhibitors had a significantly lower proportion of ATMs compared with non-users (Fig1C&D). Average CLSs were significantly higher among those with RF (48% higher; p=0.038) or a serum CRP≥10 mg/L (112% higher; p=0.001). Adipose expression of IL-6, MCP-1, MMP-9, osteopontin, C1q, and the macrophage markers CD64, CD68, and CD163 were all significantly associated with serum CRP, IL-6, DAS28 score, and duration of morning stiffness, all with Spearman correlation coefficients ranging between 0.3 and 0.7 and p-values<0.05. In a multivariable linear regression model predicting serum CRP; gender, biologic use, swollen joints, RF and/or anti-CCP seropositivity, BMI, and adipose MCP-1 expression level accounted for 63% of the explainable variability. Of this variability in CRP, adipose expression of MCP-1 independently accounted for 11% whereas swollen joints only accounted for 5%.
Conclusion: ATMs were more abundant in RA and associated with systemic inflammation and autoantibody status, suggesting possible contributions to the RA disease process. Lower levels of ATMs associated with specific RA treatments suggest that adipose tissue inflammation may be ameliorated by immunomodulation.
To cite this abstract in AMA style:
Giles JT, Ferrante AW, Broderick R, Zartoshti A, Rose J, Zhang HZ, Winchester R. Subcutaneous Adipose Tissue from Rheumatoid Arthritis Patients Is Characterized By an Abundance of Macrophages That Are Associated with Autoantibodies, Systemic Inflammation, and Immunomodulation [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/subcutaneous-adipose-tissue-from-rheumatoid-arthritis-patients-is-characterized-by-an-abundance-of-macrophages-that-are-associated-with-autoantibodies-systemic-inflammation-and-immuno/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-adipose-tissue-from-rheumatoid-arthritis-patients-is-characterized-by-an-abundance-of-macrophages-that-are-associated-with-autoantibodies-systemic-inflammation-and-immuno/