Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous studies have
shown that long-term urate-lowering therapy (ULT) is required for improvements
in gout flare frequency and tophi reduction, and that lower serum uric acid
(sUA) levels may result in greater benefit. However, optimal sUA levels to jointly
achieve these outcomes within a year using single or combination oral therapies
are uncertain, as they come mostly from observational studies. Lesinurad in
combination with a xanthine oxidase inhibitor (XOI) is highly effective in
reducing and sustaining sUA below target levels in Phase III studies. The
current analysis explored the relation between extent of reduction in sUA level
and efficacy and safety endpoints in these Phase III studies.
Methods: Patient data were combined from 3 Phase III clinical
studies on the efficacy and safety of lesinurad, a selective uric acid
reabsorption inhibitor (SURI), in combination with an XOI (allopurinol: CLEAR 1
[NCT01510158], CLEAR 2 [NCT01493531]), or febuxostat: CRYSTAL [NCT01510769]). For
the analyses, patients irrespective of treatment assignment were categorized by
their on-study median sUA level, which used all scheduled post-baseline sUAs
for each individual. Efficacy endpoints included clinical outcomes for ULT: tophus
size reduction and proportions of patients experiencing gout flares requiring
treatment (GFRT). Safety endpoints included treatment-emergent adverse events
(TEAEs).
Results: In total, 1537 patients
were eligible for efficacy analysis, including 474 patients with ≥1
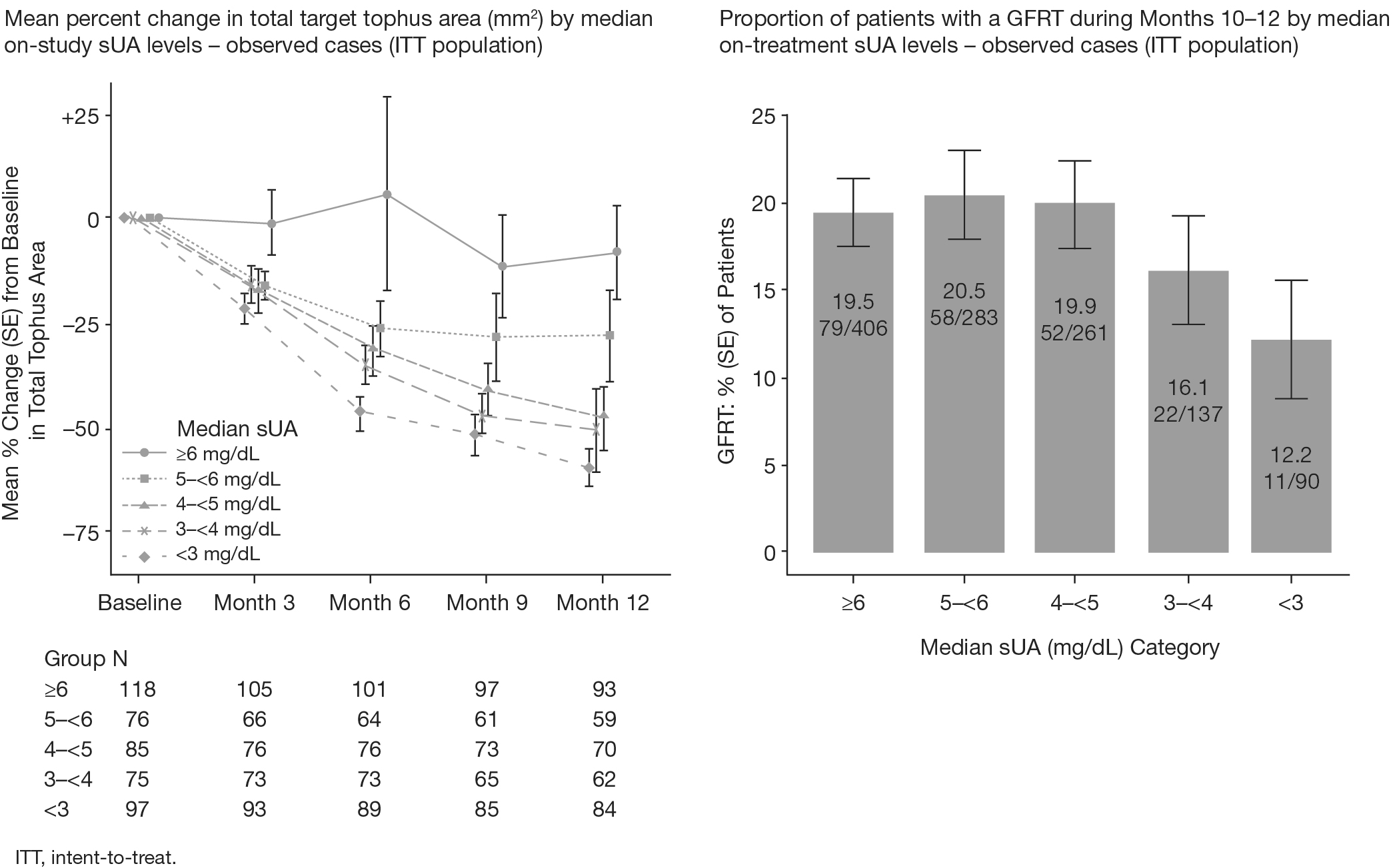
target tophi at baseline. Patients with the lowest on-study median sUA levels
achieved the greatest reduction in tophus area at Month 12 and were least
likely to have GFRT during the last quarter of the study (Figure). GFRTs occurred in 11/90 (12.2%) of patients in the <3 mg/dL sUA
subgroup and in 137/689 (19.9%) of patients in the ≥5 mg/dL subgroup (Figure). TEAEs
occurred in 82/104 (78.8%) of patients in the <3 mg/dL sUA subgroup and in
671/886 (75.7%) of patients in the ≥5 mg/dL subgroup.
Conclusion: Based on a pooled analysis
of 3 Phase III clinical studies, patients with lower median on-study sUA experienced
greater reductions in tophus area, as well as a lower likelihood of having a
gout flare requiring treatment. A similar safety profile was noted irrespective
of the median sUA levels.
To cite this abstract in AMA style:
Terkeltaub R, Perez-Ruiz F, Kopicko J, Fung M, Adler S, Storgard C, Baumgartner S, Dalbeth N. The Safety and Efficacy of Lower Serum Urate Levels: A Pooled Analysis of Gout Subjects Receiving Lesinurad and Xanthine Oxidase Inhibitors [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/the-safety-and-efficacy-of-lower-serum-urate-levels-a-pooled-analysis-of-gout-subjects-receiving-lesinurad-and-xanthine-oxidase-inhibitors/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-safety-and-efficacy-of-lower-serum-urate-levels-a-pooled-analysis-of-gout-subjects-receiving-lesinurad-and-xanthine-oxidase-inhibitors/