Session Information
Date: Monday, November 9, 2015
Title: Health Services Research II: Rheumatoid Arthritis Treatment and Healthcare Utilization
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Randomized controlled trials in RA
have reported efficacy with both triple therapy (methotrexate [MTX] +
hydroxychloroquine [HCQ] + sulfasalazine [SSZ]) and tumor necrosis factor
inhibitor and MTX (TNFi-MTX) combinations. However, observational studies of clinical practice
have been inconsistent when comparing persistence and adherence of these
combinations. This analysis compared adjusted one-year
persistence and adherence between triple and TNFi combination therapy in a
real-world setting of US Veterans.
Methods: US veterans with RA initiating triple
and TNFi combination therapy between Jan 1, 2006 and Dec 31, 2012 after
6-months of VA enrollment without prior triple or TNFi combination therapy were
evaluated for 12-months. The index date the date that the last drug (index
drug) of triple or TNFi combination was prescribed. To assure that the start of
the index drug was intended as part of combination therapy, we required that
all other drugs in the triple or TNFi combination have sequential dispensing
within 90-days after initiation of index drug. A sensitivity analysis employed
three different termination definitions of combination therapy: 1) Any drug in
the combination drugs discontinued (i.e., gap ≥ 90 days); 2) New DMARD
started, treatment reduced to monotherapy with non-biologic DMARD, or all
DMARDs discontinued; 3) Same as #2 but switching within class (TNFi or other DMARDs)
was allowed. To compare adherence, the Proportion of Days Covered (PDC)>80%
was determined for each drug and the drugs in combination over the12 months.
Propensity score matched weights were used to balance covariates when comparing
risk ratios for each outcome among triple and combination therapy.
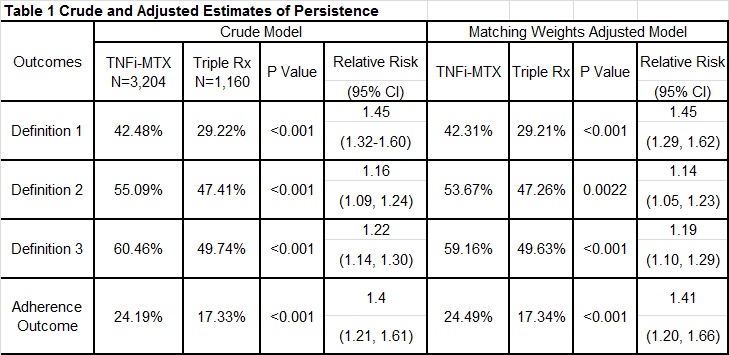
Results: A total of 3,204 TNFi-MTX and 1,160
triple therapy patients met the eligibility criteria (mean [SD] age at index 61
[11] vs. 62 [10], 16.0% vs. 9.0% female). After 12 months, persistence in the
TNFi-MTX arm was significantly greater by all three approaches in both crude
and propensity score weighted models (table1); favoring TNFi combination
treatment. Twelve-month adherence to TNFi-MTX was approximately 41% higher than
adherence to triple therapy [24% vs. 17%, RR: 1.41 (95%CI: 1.20,1.66)].
Conclusion: US Veterans initiating TNFi-MTX
therapy consistently showed significantly greater persistence and
adherence than those receiving triple therapy in clinical practice. Given
differences between treatment strategies in terms of cost, tolerability, and
patient preference, additional research focused on identifying factors that
account for the observed differences in persistence and adherence will be
important in informing RA management.
Disclosure: B. Sauer, Amgen, 2; C. C. Teng, Amgen, 2; J. Leng, Amgen, 2; T. R. Mikuls, Roche /Genetech, 2,Pfizer Inc, 5; J. R. Curtis, Roche, UCB Pharma, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo,AbbVie, 2,Roche, UCB Pharma, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo,AbbVie, 5; B. S. Stolshek, Amgen Inc., 3,Amgen Inc., 1; D. Tang, Amgen Inc., 3,Amgen Inc., 1; G. W. Cannon, Amgen, 2.
To cite this abstract in AMA style:
Sauer B, Teng CC, Leng J, Mikuls TR, Curtis JR, Stolshek BS, Tang D, Cannon GW. Higher Persistence and Adherence with Combination Therapy with Tumor Necrosis Factor Inhibitor+Methotrexate Combination Versus Triple Therapy in US Veterans with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/higher-persistence-and-adherence-with-combination-therapy-with-tumor-necrosis-factor-inhibitormethotrexate-combination-versus-triple-therapy-in-us-veterans-with-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/higher-persistence-and-adherence-with-combination-therapy-with-tumor-necrosis-factor-inhibitormethotrexate-combination-versus-triple-therapy-in-us-veterans-with-rheumatoid-arthritis/