Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tofacitinib is a novel, oral Janus kinase inhibitor being investigated as a targeted immunomodulator and disease-modifying therapy for RA. Clinical guidelines recommend the use of influenza and pneumococcal vaccines in patients with RA; however, the effect of tofacitinib on vaccine immunogenicity is unknown.
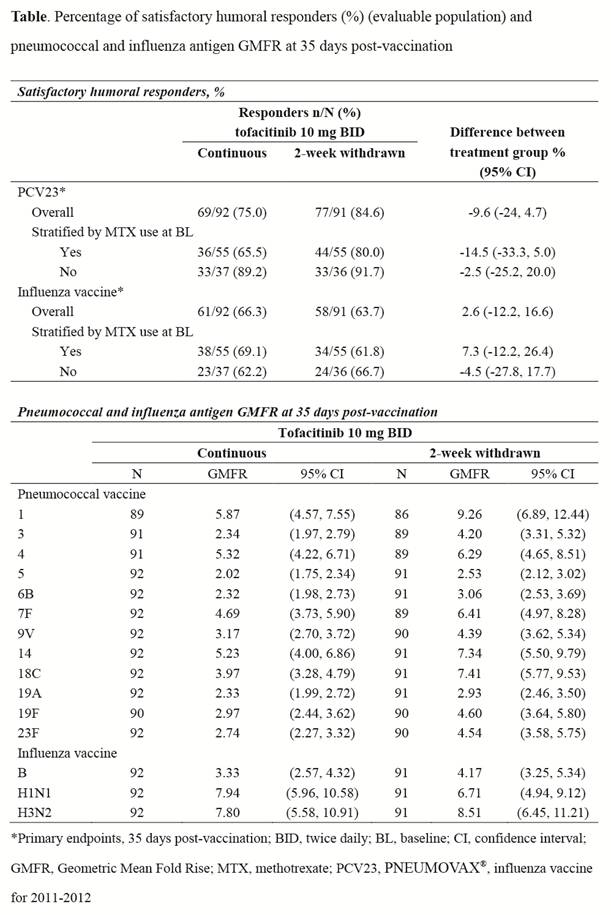
Methods: Patients with RA taking tofacitinib 10 mg twice daily (BID) were recruited from an open-label, long-term extension trial (NCT00413699). In this vaccine substudy, participants were randomized into 2 groups and stratified by methotrexate (MTX) use: 1) those who continued tofacitinib 10 mg BID during and after randomization (“continuous”); or 2) those who interrupted tofacitinib use for 1 week prior to and 1 week after vaccination (“withdrawn”). Vaccination baseline serum pneumococcal and influenza antibody titers were measured and all patients were vaccinated with the 2011-2012 influenza vaccine and PNEUMOVAX® 23 (PCV23, Merck & Co., Inc) at 1 week post-randomization; antibody titers were measured at 35 days post-vaccination. The primary endpoint was the proportion of continuous and withdrawn patients who achieved a satisfactory humoral response to (a) pneumococcal vaccine (≥2-fold increase in antibody concentrations against ≥6 of 12 pneumococcal antigens [serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 19A, 19F, 23F, 18C]) and (b) influenza vaccine (≥4-fold increase in antibody titers against at least 2 of 3 influenza antigens). Secondary endpoints included comparison of pre- and post-vaccine hemaglutination titers (HI), and influenza and pneumococcal serotype-specific Geometric Mean Fold Rise (GMFR) between groups.
Results: Of 199 patients enrolled, 183 (continuous, N=92; withdrawn, N=91) completed vaccination and antibody titer evaluations and were included in the analysis. The proportion of patients achieving a satisfactory humoral response to pneumococcal and influenza vaccines was similar between patients who continued tofacitinib and those who were temporarily withdrawn (Table). Post-vaccination pneumococcal antigen GMFRs trended higher in patients withdrawn from tofacitinib, while GMFRs were similar between groups for influenza (Table). Protective influenza HI titers (≥1:40 influenza antibody titer in ≥2 of three antigens) were similar for continuous (75%) and withdrawn patients (82%) at 35 days post-vaccination.
Conclusion: Among patients with RA, continuous tofacitinib use did not significantly impair overall responsiveness to pneumococcal and influenza vaccines, although the pneumococcal antigen GMFRs trended higher in patients who discontinued tofacitinib. A study limitation is the absence of a MTX-only control group or group lacking tofacitinib exposure. These data suggest that it is not necessary to discontinue tofacitinib in order to attain meaningful responses to pneumococcal and influenza vaccines.
Disclosure:
K. L. Winthrop,
Oxford Immunotech; Pfizer,
2,
Abbott; Pfizer; UCB; Amgen; Cellestis,
5;
A. Racewicz,
None;
E. B. Lee,
Pfizer Inc.,
5;
B. Wilkinson,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
S. H. Zwillich,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
K. Soma,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
S. Rottinghaus,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
T. Kawabata,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
R. Riese,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
S. Wood,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
J. Bradley,
Pfizer Inc,
1,
Pfizer Inc,
3;
C. O. Bingham III,
Pfizer Inc.,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-influenza-and-pneumococcal-vaccine-responses-in-patients-with-rheumatoid-arthritis-receiving-tofacitinib/