Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The impact of childhood-onset lupus (cSLE) and its treatment on quality of life (QoL) has not been systematically established. The Patient Reported Outcomes Measurement Information System (PROMIS™, http://nihpromis.org) is a publicly available system to measure patient reported outcomes that features electronic data collection. Although several legacy QoL measures have been validated for cSLE, each is longer than the PROMIS™ Pediatric Short Forms (Short Forms) which are ≤ 10 items each. The objective of this study was to investigate the feasibility and construct validity of the Short Forms in cSLE in a clinical setting.
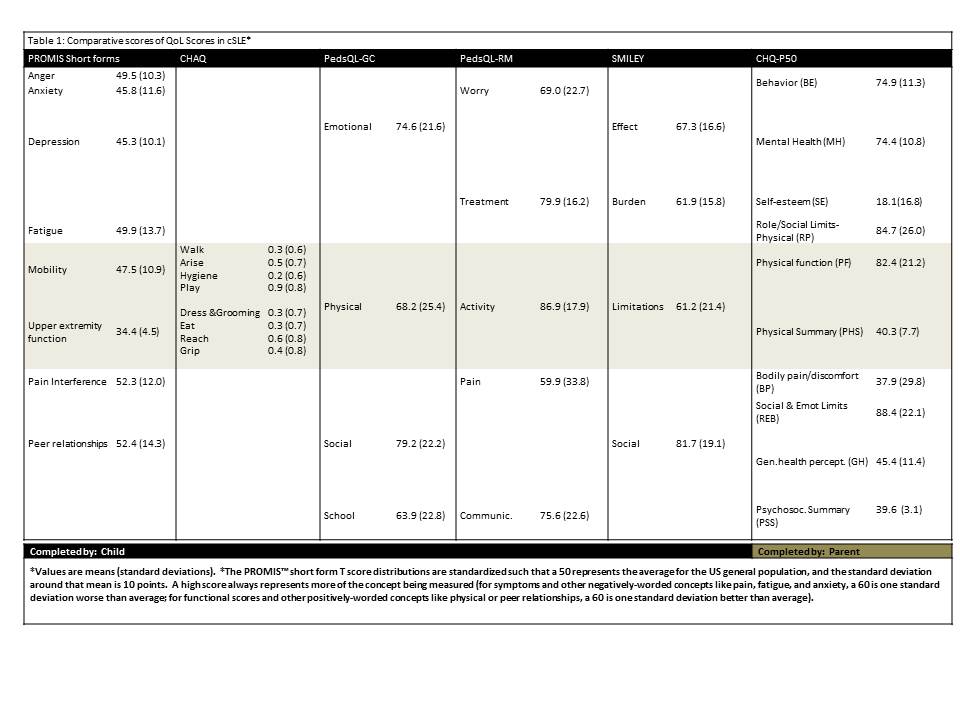
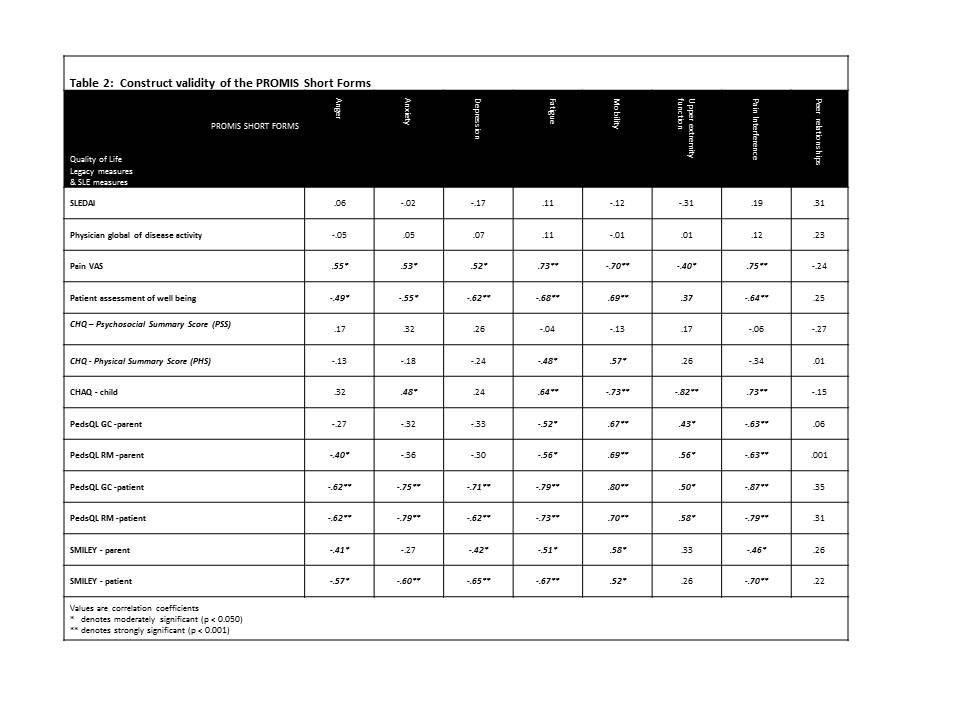
Methods: As part of an ongoing project at two sites, 30 of 100 projected patients completed the Pediatric PROMIS™ Short Forms (Anger, Anxiety, Depression, Fatigue, Mobility, Upper Extremity, Pain Interference, Peer Relationships) and legacy QoL measures (Pediatric Quality of Life Inventory™ Generic Core [GC] & Rheumatology Modules [RM], Simple Measure of Impact of Lupus Erythematosus in Youngsters [SMILEY], Childhood Health Assessment Questionnaire [CHAQ], Child Health Questionnaire [CHQ], pain and well-being visual analog scales [VAS]). Questionnaire scores were compared and Pearson correlation analysis was performed in support of the construct validity of the Short Forms when used in cSLE.
Results: Participants (80% female; 48% White, 52% Black or Asian) had a mean age of 15.8 yrs (SD 2.1) and mean SLEDAI score of 5.8 (SD 4.57). No problems were encountered with completion of all PROMIS™ Short Forms (mean score = 50, clinically relevant score difference = 10) which required 5-10 minutes in total (legacy QoL tools >15 min each). On average, cSLE patients showed clinically relevant decrease in the Short Form assessing upper extremity function compared to healthy children, while the other QoL domains were less affected (Table 1). This is also supported by the scores of the CHQ-PHS and GC. Concurrent validity of the Short Forms is supported by moderate correlations with the scores of various legacy measures. Comparison to cohorts of healthy children and those with juvenile arthritis will be provided.
Conclusion: Preliminary analysis supports QoL measurement using the PROMIS™ Short Forms as feasible and concurrently valid. If confirmed in the larger sample, clinicians treating cSLE will be able to utilize PROMIS™ measures for a more efficient patient self-report of health outcomes, taking advantage of easy interpretation of scores and changes in scores, thereby, reducing respondent burden and making QoL assessment feasible in both research and clinical care settings.
Disclosure:
A. J. Greenler,
None;
L. E. Schanberg,
None;
M. P. Flannery,
None;
S. Nelson,
None;
J. Wootton,
None;
E. M. Morgan DeWitt,
None;
H. Brunner,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-promis-modules-for-use-in-childhood-onset-lupus/