Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Randomized clinical trials have shown that if a patient is in sustained remission, biological disease-modifying anti rheumatic drugs (bDMARDs) therapy can be tapered in rheumatoid arthritis (RA). However, little is known about down-titration of bDMARDs in the real world. We retrospectively analyzed our cohort to determine the frequency and predictive factors associated with the successful down-titration of bDMARD in RA patients in our two university hospitals.
Methods: This study included consecutive RA patients who fulfilled 1987 ACR and/or 2010 ACR/EULAR classification criteria and treated with bDMARD (infliximab, adalimumab, etanercept, golimumab, certolizumab-pegol, tocilizumab, and abatacept) for longer than 6 months. The patients receiving stable and standard dose treatment were defined as SD group, while the patients receiving down-titration were defined as DT group. We retrospectively reviewed clinical charts and compared the route of administration, concomitant medication use, laboratory data, X-ray findings and patient characteristics at the beginning of bDMARD between SD group and DT group. Statistical analyses were performed using univariate and multivariate analysis.
Results: A total of 347 patients (age 62.5±13.8 years old, female 83.6%, disease duration 12.3±8.5 years) were included in this analysis, 255 (73.5%) and 92 (26.5%) patients belonged to SD and DT groups, respectively. Five (1.4%) patients experienced disease flare after down-titration of bDMARD after 8.4±5.7months. There was no significant difference between SD group and DT group in disease duration, route of administration, prevalence of the anti-citrullinated protein antibody and the rheumatoid factor, TJC, SJC, PGA, DAS28-ESR, and X-ray at baseline. In the univariate analyses of baseline data, there were statistically significant differences in the rates of bDMARD naïve patients, age at onset, age at the beginning of bDMARD, concomitant oral corticosteroid use, and CRP levels between SD group and DT group (57.1% vs 76.4%, p = 0.001, 51.0 ± 14.6 vs 47.1 ± 14.9, p = 0.03, 59.6 ± 13.7 vs 55.5 ± 14.6, p = 0.02, 43.1% vs 34.8%, p=0.04, and 1.9 ± 2.5 vs 1.1 ± 1.3, p = 0.03, respectively). Multivariate statistical analysis revealed the low level of serum CRP at baseline were associated with successful down-titration (p = 0.03, odds ratio = 0.81, 95% CI: 0.663-0.990).
Conclusion: Down-titration of biologics might be achieved in RA patients who have the following factors at the beginning of bDMARD: bDMARD naïve, younger age, no concomitant use of corticosteroid, and low level of serum CRP.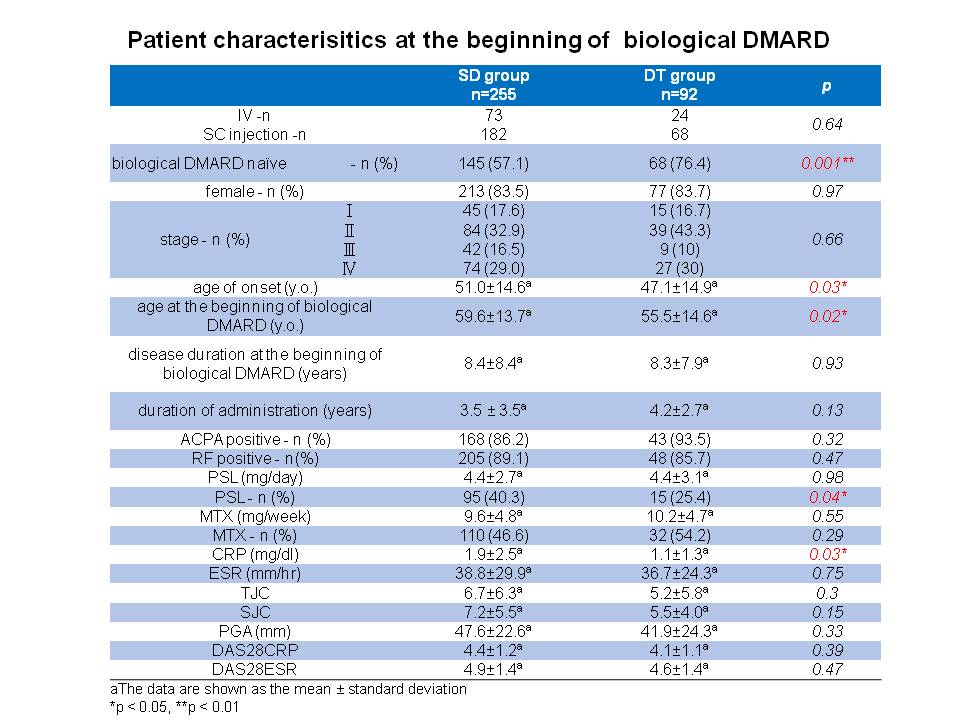
To cite this abstract in AMA style:
Komiya T, Minegishi-Takase K, Sakurai N, Sato Y, Nagai H, Hamada N, Sugiyama Y, Tsuchida N, Soejima Y, Kunishita Y, Nakano H, Kishimoto D, Kobayashi K, Kamiyama R, Yoshimi R, Asami Y, Kirino Y, Ohno S, Nakajima H. Predictive Factors Associated with Successful Down-Titration of Biologics for Rheumatoid Arthritis Patients in Clinical Practice [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/predictive-factors-associated-with-successful-down-titration-of-biologics-for-rheumatoid-arthritis-patients-in-clinical-practice/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictive-factors-associated-with-successful-down-titration-of-biologics-for-rheumatoid-arthritis-patients-in-clinical-practice/
