Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The
diagnosis of polyarticular JIA (pJIA) is made in childhood but the disease and
the diagnosis are carried into adulthood. Adults with pJIA have heterogeneous
clinical manifestations, many of which differ from rheumatoid arthritis. These
adults warrant a tailored approach for optimal management of pJIA. The
objective of this study was to assess adalimumab (ADA) effectiveness and safety
in pts with pJIA who turned 18 years (yrs) of age anytime during Study DE038.
Methods: This was
a multicenter, phase 3, randomized, double-blind (DB), stratified study in
children (4 to 17 yrs at baseline) with pJIA treated with or without MTX prior
to the study entry. Pts in the MTX arm were treated concomitantly with MTX
during the entire study duration. Pts in
the non-MTX arm were either naïve to or withdrawn from MTX at least 2 weeks
(wks) prior to study drug administration. The study consisted of 16 wk open
label lead-in, a 32 wk DB phase and an open label (OL) extension phase. This
post-hoc analysis assessed outcomes during the OLE for pts who turned 18 yrs anytime
during the course of study in comparison to pts who remained under 18 yrs
during their study participation. Twenty sevenjoint juvenile arthritis disease
activity score (JADAS27), based on C-reactive protein (CRP) response was
measured upto 312 wks of the OLE. Adverse events (AEs) were also monitored and
compared between the two age groups throughout the study duration.
Results: 171 pts
with pJIA between 4-17 yrs of age at the time of study entry were enrolled. Among
them, 51(30%) turned 18 yrs of age during the course of their participation in
the study and were compared to the remaining 120 pts that remained <18 yrs throughout the study participation.
There were (42; <18 yrs) vs (46;
≥18 yrs) pts who received at least 4 yrs of ADA. The mean age at the time
of study enrollment was 14.6 yrs vs 9.8 yrs in pts ≥18 yrs and <18 yrs
respectively. The majority of pts were white (90.2% vs 92.5%) and female (74.5%;
≥18 yrs) vs 80.8%; <18 yrs). The subgroup of 51 pts that turned 18 yrs
had a mean age of 19.8 ± 1.5 yrs at the time of their last dose of ADA in the
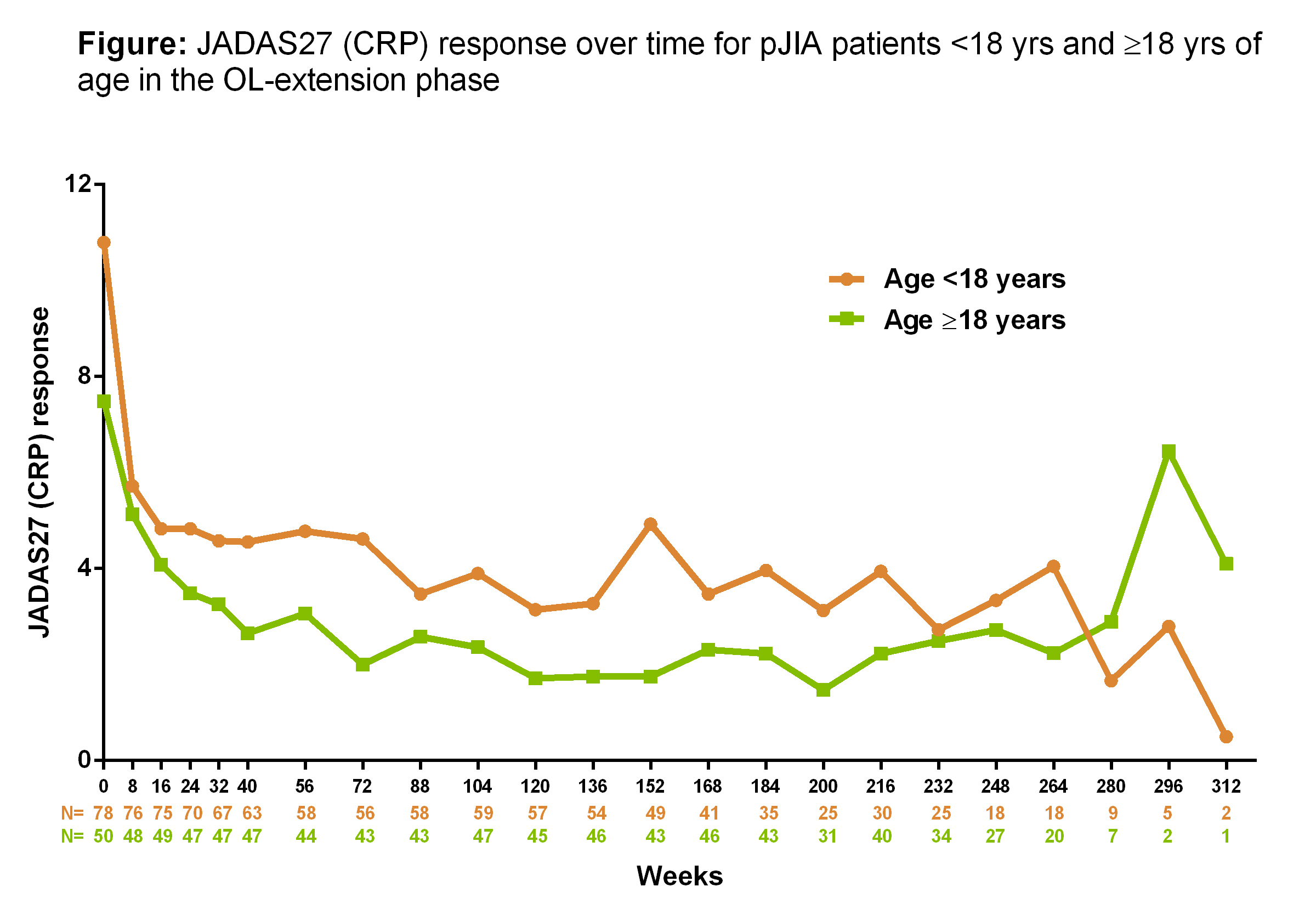
study. The JADAS27(CRP)
response was measured from baseline up to 312 wks and demonstrated maintainance
of efficacy (p<0.05)from week 72 onwards, irrespective of age (Figure). The overall rate of AEs was
lower in pts ≥18 yrs than pts <18 yrs (432 [95% CI, 407.6 – 458.6] vs 701 [95% CI, 672.1 –
731.0] events/100 pt-y) respectively.
Conclusion: The efficacy results based
on the JASDAS27(CRP)responses demonstrated maintenance
of efficacy over time for both age groups. Safety data showed the overall rate of AEs was lower in pts ≥18 yrs than pts
<18 yrs. No new safety signals were observed; thus the safety profile of ADA
remained unchanged. The findings from this study support a favorable
benefit-risk profile of for the ADA treatment in adult pts (≥18 yrs of age) with moderately to severely active pJIA.
To cite this abstract in AMA style:
Lovell D, Ruperto N, Reiff A, Jung L, Jarosova K, Quartier P, Sandborg C, Bohnsack JF, Elewaut D, Foeldvari I, Rovensky J, Giannini EH, Varothai NA, Kalabic J, Martini A. Efficacy and Safety of Adalimumab in Adult Patients with Polyarticular Juvenille Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-adalimumab-in-adult-patients-with-polyarticular-juvenille-idiopathic-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-adalimumab-in-adult-patients-with-polyarticular-juvenille-idiopathic-arthritis/