Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Zasocitinib (TAK-279) is a highly selective, oral, allosteric tyrosine kinase 2 (TYK2) inhibitor. In a phase 2b trial in patients with active psoriatic arthritis (PsA; NCT05153148), significantly more patients treated with zasocitinib achieved ACR20 responses at Week 12 than with placebo (15 mg [53.3%], 30 mg [54.2%], placebo [29.2%]; p = 0.002).1 Here, additional data on skin and overall disease activity are presented.
Methods: This was a randomized, multicenter, double-blind, placebo-controlled study. Eligible patients were aged ≥ 18 years, with PsA symptoms for ≥ 6 months prior to screening, met CASPAR criteria, and had ≥ 3 tender and ≥ 3 swollen joint counts at enrollment despite prior treatment with biologics, DMARDs or NSAIDs. If on concurrent PsA treatments (permitted therapies were pre-defined in the study protocol), patients remained on stable doses throughout the study. Patients were randomized (1:1:1:1) to oral zasocitinib (5, 15 or 30 mg) or placebo, once daily for 12 weeks. Outcomes included 75/90/100% improvements from baseline in psoriasis area and severity index (PASI 75/90/100), change from baseline in PASI in patients with ≥ 3% psoriasis (PsO) body surface area (BSA) at baseline, Physician Global Assessment of PsO (PhGA-PsO) response (0/1 and a ≥ 2-point improvement from baseline in patients with a PhGA-PsO score ≥ 2 at baseline) and achievement of minimal disease activity (MDA). Differences were assessed using a Mantel–Haenszel test (binary endpoints) and a mixed model for repeated measures (continuous endpoints); p values were nominal.
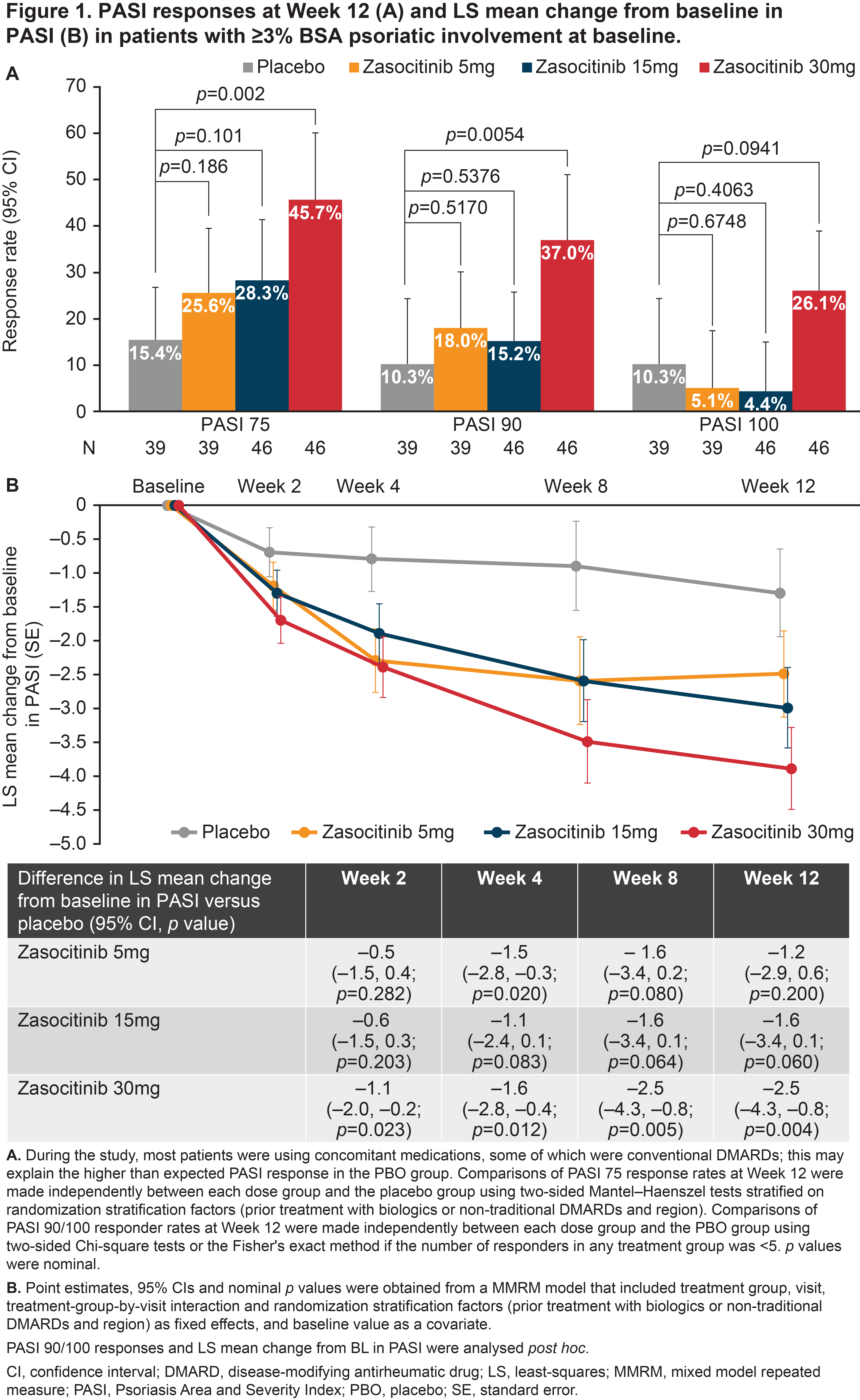
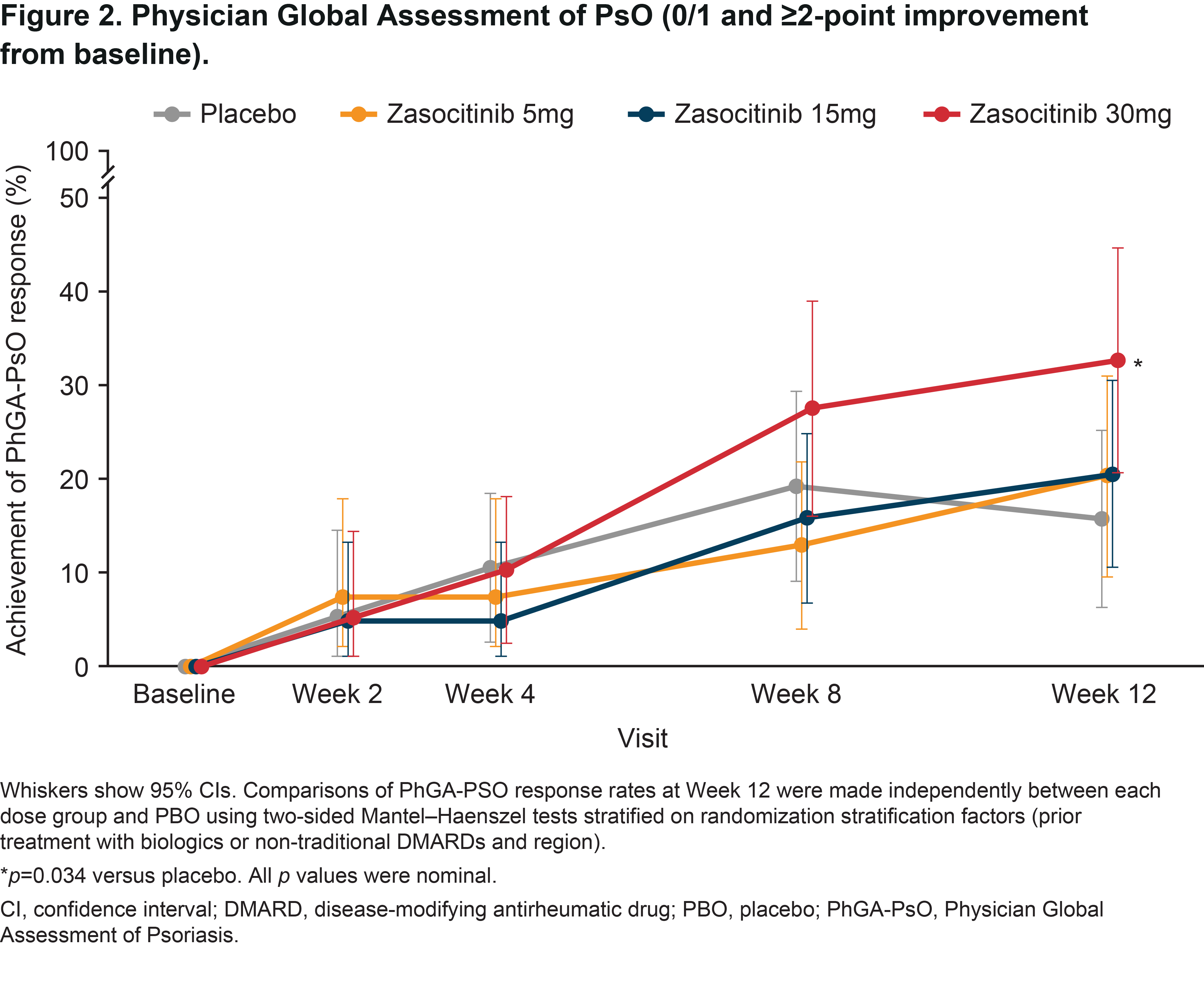
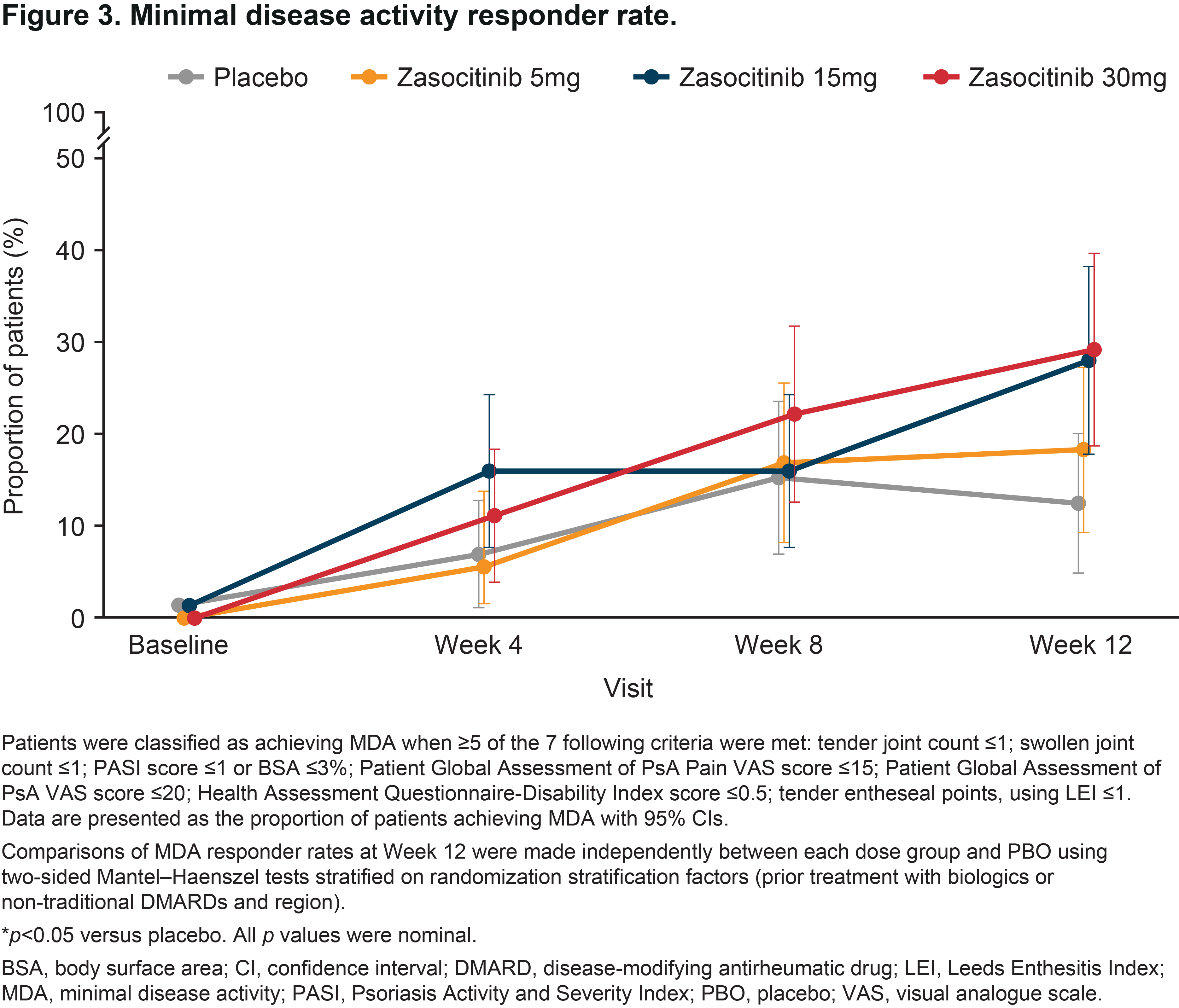
Results: Overall, 290 patients were randomized. Baseline demographics and disease characteristics were similar between groups; 58.6% had PsO BSA ≥ 3% (mean [SD] baseline PASI, 6.2 [5.5]). At baseline, 59.7, 63.4, 61.3 and 65.3% of patients were receiving concomitant traditional DMARDs in the placebo and zasocitinib 5, 15 and 30 mg groups, respectively. In the 30 mg group, Week 12 PASI responses were substantially higher than with placebo or lower zasocitinib doses (Figure 1A). Reductions in least-squares mean (LSM) change from baseline in PASI were greater for zasocitinib 30 mg than placebo from Week 2 (–1.1 vs placebo; 95% CI: –2.0, –0.2; p = 0.023) and maintained through Week 12 (–2.5 vs placebo; –4.3, –0.8; p = 0.004, Figure 1B). A substantially higher number of patients also achieved a PhGA-PsO response with zasocitinib 30mg than with placebo at Week 12 (32.8% vs 15.8%; p = 0.034, Figure 2). Higher doses of zasocitinib resulted in higher rates of MDA at Week 12 (15 mg, 28.0%; 30 mg, 29.2%; placebo, 12.5%; both p < 0.05; Figure 3). Zasocitinib was well tolerated with a safety profile consistent with that seen in the phase 2b PsO study.2
Conclusion: Zasocitinib 30 mg was well tolerated and demonstrated robust efficacy across all skin endpoints assessed versus placebo. Skin responses were evident from Week 2 onwards, and more patients treated with zasocitinib 30 mg achieved total or near-total clearance of PsO lesions at Week 12 versus placebo. Higher doses of zasocitinib (15 and 30 mg) also achieved higher rates of MDA indicating meaningful improvement in other core PsA domains.
References:
1. Kivitz A, et al. Arthritis Rheumatol 2023;75(Suppl 9).
2. Armstrong A, et al. AAD 2023.
To cite this abstract in AMA style:
Gottlieb A, Muensterman E, Kivitz A, Dokoupilova E, Lertratanakul A, Hong T, Chen J, Baraliakos X. Zasocitinib (TAK-279), a Highly Selective Oral Tyrosine Kinase 2 (TYK2) Inhibitor, Elicits Early Skin Responses and Minimal Disease Activity in Patients with Active Psoriatic Arthritis: Results from a Randomized Phase 2b Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/zasocitinib-tak-279-a-highly-selective-oral-tyrosine-kinase-2-tyk2-inhibitor-elicits-early-skin-responses-and-minimal-disease-activity-in-patients-with-active-psoriatic-arthritis-results-from-a/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/zasocitinib-tak-279-a-highly-selective-oral-tyrosine-kinase-2-tyk2-inhibitor-elicits-early-skin-responses-and-minimal-disease-activity-in-patients-with-active-psoriatic-arthritis-results-from-a/