Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Yellow fever (YF) immunization is not routinely performed in juvenile autoimmune rheumatic disease (ARD) patients. However, during the recent epidemic campaign in state of Sao Paulo this live attenuated vaccine was indicated for patients under low immunosuppression (PAHO and WHO). There are no data regarding YFV safety in juvenile ARD patients. Therefore, the aim of this study was to evaluate the short-term safety of immunization with fractional YFV in juvenile ARD.
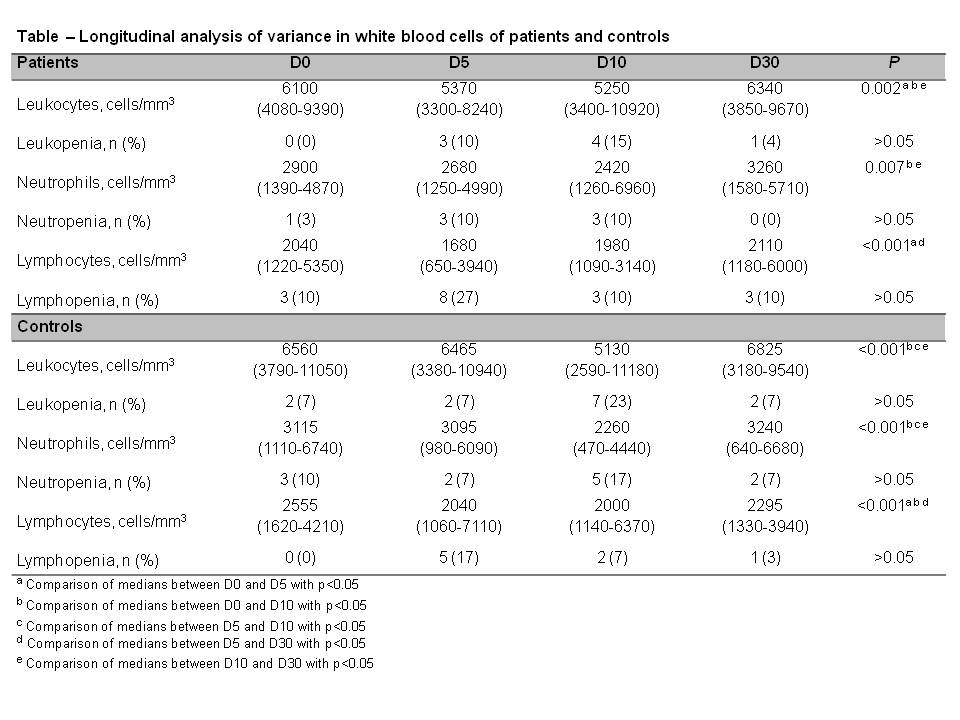
Methods: Thirty pediatric patients with inactive rheumatic diseases treated with low dose immunosuppression (sulfasalazine, prednisone≤0.5mg/Kg/day or 20mg/day, MTX≤0.4mg/Kg/week or 20mg/week, leflunomide) and living in high risk area [16 juvenile idiopathic arthritis, 6 Henoch-Schönlein purpura, 4 juvenile systemic lupus erythematosus, 3 juvenile dermatomyositis and 1 juvenile systemic sclerosis] and 30 healthy controls with comparable ages were vaccinated with a fractional dose of the 17DD YF vaccine [one fifth (0.1 ml) of the standard dose]. All participants were evaluated pre-vaccination (D0) and after 5 days (D5), 10 days (D10) and 30 days (D30) for clinical and laboratory parameters (AST, ALT, complete blood count, CRP). Disease activity was evaluated according to specific tools for each juvenile ARD at D0 and D30. A rigorous follow-up of adverse events was performed during the first 30 days after vaccination. Serious adverse events were defined as those resulting in hospitalization or death.
Results: Patients and controls had comparable median age [12.4(6.3-18.2) vs. 12(6.9-18.7) years, P= 0.25]. Disease activity parameters of ARD patients (JADAS-71, SLEDAI-2K, CMAS, DAS, MMT, ESR and CRP) remained stable 30 days after YFV (P>0.05). Patients and controls reported only mild symptoms during follow-up, with comparable frequencies of fever, muscle pain, abdominal pain, nausea and diarrhea (P>0.05). Both groups presented similar median white blood cells counts kinetics with transient decreases in leukocytes and neutrophils levels in D10, followed by full recovery to baseline levels in D30 (P<0.05) (Table1). For lymphocytes the decrease occurred at D5, with complete recovery in D30 (P<0.05). Of note, new onset leukopenia (<4,000mm3), neutropenia (<1,500mm3) and lymphopenia (<1,500mm3) were rarely observed in these patients after vaccine (Table1). Liver enzymes alterations and acute kidney injury were not observed in patients and healthy controls, and none of the patients and controls had serious adverse events.
Conclusion: Fractional dose of 17DD YFV was safe and well tolerated in juvenile ARD. We further demonstrated that this vaccine did not trigger disease flare. Therefore, this study may contribute to future recommendation for YF vaccine in pediatric patients, under low immunosuppression, living or travelling in endemic areas. (ClinicalTrials.gov, NCT03430388)
To cite this abstract in AMA style:
Aikawa NE, Balbi VA, Tonacio AC, Sallum AME, Campos LMA, Kozu KT, Vendramini MB, Fontoura N, Sartori AM, Antonangelo L, Silva CA, Bonfa E. Yellow Fever Vaccination in Brazil: Short-Term Safety in Pediatric Autoimmune Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/yellow-fever-vaccination-in-brazil-short-term-safety-in-pediatric-autoimmune-rheumatic-diseases/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/yellow-fever-vaccination-in-brazil-short-term-safety-in-pediatric-autoimmune-rheumatic-diseases/