Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Belimumab (BEL) is a recombinant IgG1λ monoclonal antibody that is approved for treatment of systemic lupus erythematosus (SLE). Although clinical studies of BEL have demonstrated a favorable benefit–risk profile, varying incidence rates of mortality and adverse events of special interest, including malignancies, warrant further consideration. The Belimumab Assessment of Safety in SLE (BASE) placebo- (PBO)-controlled trial was conducted to assess long-term safety following BEL exposure.1
Methods: This was a post-treatment follow-up of the Phase 4, double-blind BASE study (GSK Study BEL115467; NCT01705977).1 A total of 4003 adults with active, autoantibody positive SLE received BEL (10 mg/kg IV) or PBO, plus standard therapy (ST) for 48 weeks. Following the treatment period, patients entered a Year 2−5 follow-up period in which they received physician-directed ST. All patients were contacted annually by telephone, including patients who discontinued treatment during the study. Mortality and new primary malignancies (including nonmelanoma skin cancer [NMSC]) were the only endpoints collected and rates were summarised. We present the data for the Year-3 follow-up by treatment received during the 52-week double-blind treatment period (Year 1).
Results: Baseline characteristics at the start of the 52-week treatment for the Year-3 follow-up population (N=3266) were similar to those of the Year-1 double-blind study population (N=4003). By the Year-3 follow-up, cumulatively 12.0% and 10.9% of patients in the original BEL and PBO Year-1 treatment groups had received BEL as part of physician-directed care, respectively. In total (for both treatment groups), crude mortality rates were similar across Year 1 (0.87%), Year 2 (0.89%), and Year 3 (0.80%), whilst crude malignancy rates for Year 3 (0.52%) were numerically higher than Year 2 (0.30%), but similar to Year 1 (0.47%) (Table 1). Mortality and malignancy rates were lower in the BEL versus PBO Year-1 treatment group. Cumulative rates are shown in Table 2.
Conclusion: Post-treatment follow-up results in Year 3 from BASE, the largest study of patients with SLE to date, provide continued support for the safety profile of BEL and remained consistent with the Year-2 follow-up data. No new safety concerns for BEL were identified in patients with active, autoantibody-positive SLE receiving ST.
Funding: GSK
References:
1Sheikh SZ, et al. Lancet Rheum 2020 (ePub ahead of print) doi.org/10.1016/S2665-9913(20)30355-6
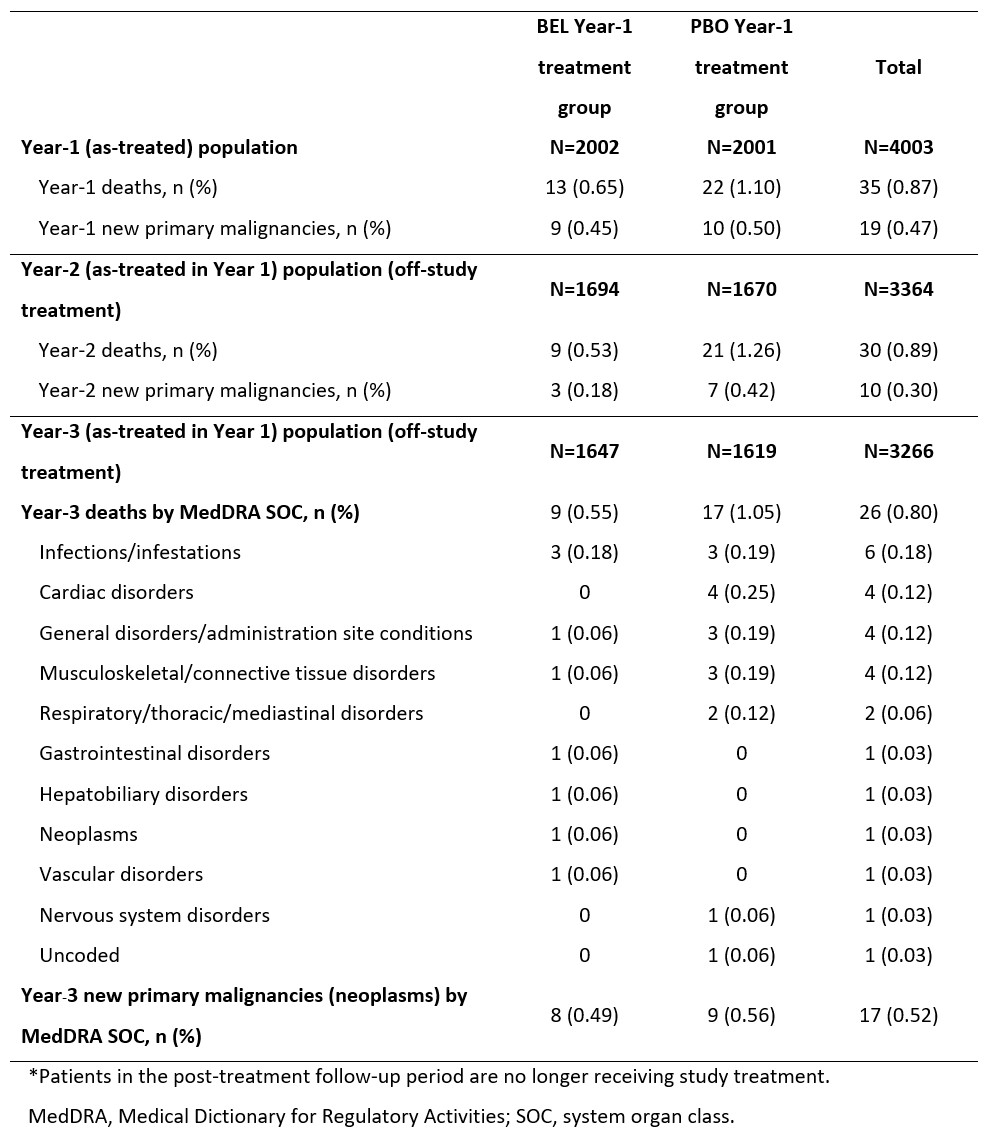 Table 1. Year_1, Year_2 and Year_3 post-treatment* follow-up mortality and new primary malignancy rates by study treatment during Year 1
Table 1. Year_1, Year_2 and Year_3 post-treatment* follow-up mortality and new primary malignancy rates by study treatment during Year 1
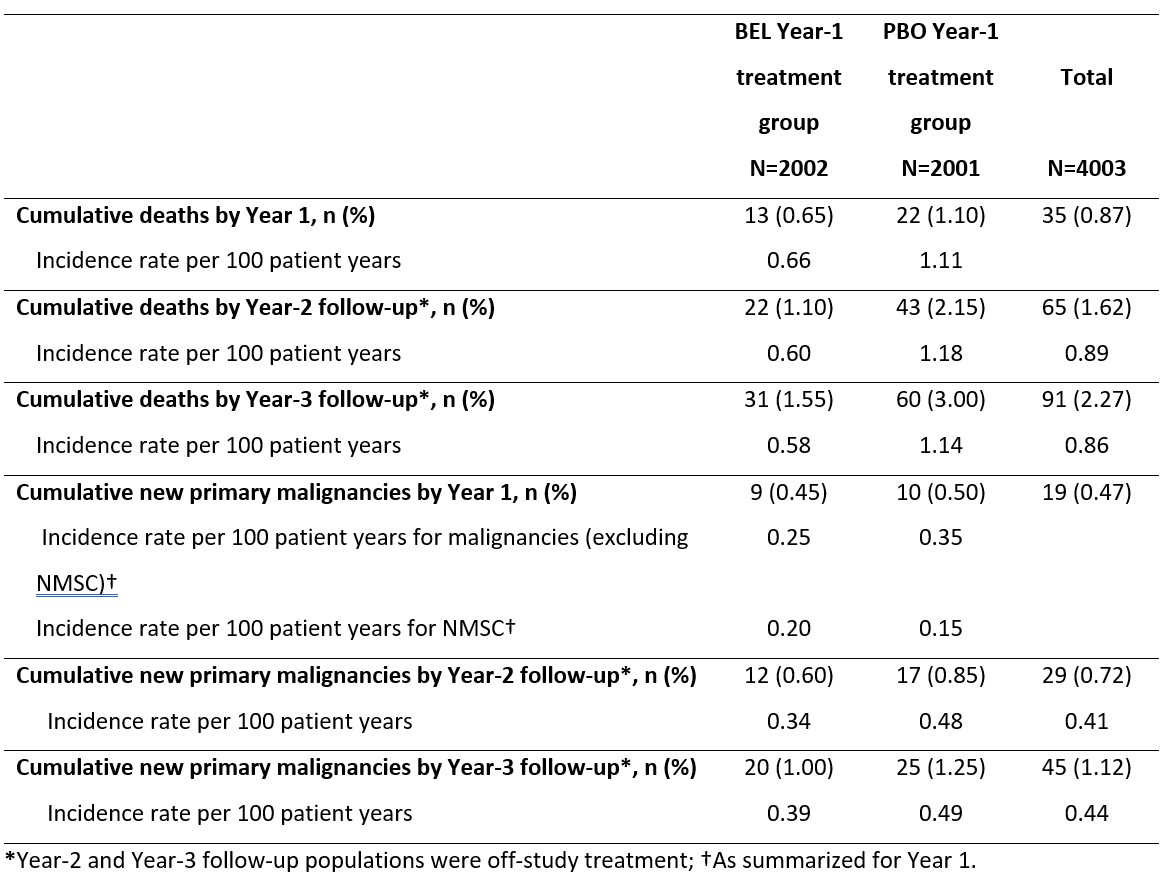 Table 2. Cumulative deaths and new primary malignancies by follow-up year
Table 2. Cumulative deaths and new primary malignancies by follow-up year
To cite this abstract in AMA style:
Sheikh S, Wei C, Tegzova D, Stohl W, Acayaba de Toledo R, Mucenic T, Abello Banfi M, Maksimowicz-McKinnon K, Abud-Mendoza C, Navarra S, Garcia M, Garcia-De La Torre I, Kurrasch R, Fernandes S, Harris J, Muzaffar S, Fox N, Liu A, Quasny H, Roth D. Year-3 Observational Follow-up of Belimumab Safety (Mortality and Malignancies) in Patients with SLE Who Completed a Phase 4, 52-Week, Randomized, Double-Blind Placebo-Controlled Safety Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/year-3-observational-follow-up-of-belimumab-safety-mortality-and-malignancies-in-patients-with-sle-who-completed-a-phase-4-52-week-randomized-double-blind-placebo-controlled-safety-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/year-3-observational-follow-up-of-belimumab-safety-mortality-and-malignancies-in-patients-with-sle-who-completed-a-phase-4-52-week-randomized-double-blind-placebo-controlled-safety-study/
