Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: SLE is a chronic autoimmune disease characterized by diverse clinical manifestations and flares associated with organ damage, resulting in substantial short-term management costs. Results from the BLISS-SC trial demonstrated that subcutaneous (SC) belimumab plus standard of care (SoC) compared with placebo plus SoC was associated with significant reductions in flares.1 This study evaluated whether reductions in flares were associated with lower healthcare costs when comparing belimumab and placebo in the United States (US).
Methods: This retrospective, observational, post hoc economic analysis (GSK study 207134) from the US payer perspective used data from BLISS-SC, a 52-week, Phase 3, randomized trial (NCT01484496), comparing weekly belimumab 200 mg SC plus SoC and placebo plus SoC in adults with active SLE. The primary endpoint was the cost of treating severe flares; the secondary endpoint was the cost of treating flares of any severity. The time horizon was 52 weeks. Unit cost per flare (2017 US$) was calculated from an analysis using an algorithm to identify flares in US claims data.2 An event-based costing methodology was subsequently applied to flare events observed in BLISS-SC, allowing for a comparison of costs associated with treating flares in the belimumab group versus placebo. Generalized linear models with negative binominal distribution were used to predict adjusted rates of severe and mild/moderate flares per patient. Adjusted rates of flares per patient were then multiplied by the flare unit cost (from claims data) to obtain adjusted costs associated with treatment of severe and mild/moderate flares. The mean (95% confidence interval [CI]) adjusted costs of any severity flares were computed by adding the estimated costs of severe and mild/moderate flares per patient.
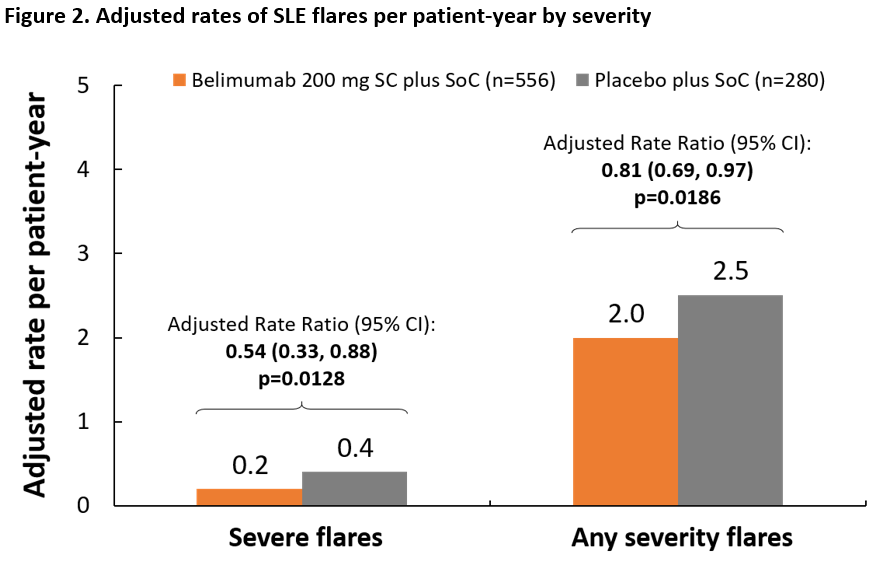
Results: 836 patients from the intent-to-treat BLISS-SC population were included (belimumab plus SoC, n=556; placebo plus SoC, n=280); mean (standard deviation, SD) age was 38.6 (12.3) years; 94.4% were female. The mean (SD) unit cost was $9,273 ($38,800) per severe flare and $2,303 ($7,821) per mild/moderate flare. Mean adjusted predicted costs associated with treating flares during follow-up were significantly lower in the belimumab plus SoC group than in the placebo plus SoC group (severe flares, $927 lower, p< 0.001; flares of any severity, $1,379 lower, p< 0.001) (Figure 1). The adjusted rate of severe flares per patient-year was lower in the belimumab plus SoC versus the placebo plus SoC group (Figure 2).
Conclusion: This post hoc analysis of BLISS-SC data showed significantly lower costs of treating flares in patients with SLE treated with SC belimumab plus SoC compared with placebo plus SoC. Although the study is representative of a select clinical trial population and may differ from real-world patients with SLE, these results may help inform decision makers considering the introduction of SC belimumab to their healthcare system.
Study funding: GSK. Medical writing support: Gosia Carless, PhD, Fishawack Indicia Ltd, UK (funded by GSK).
1Stohl W, et al. Arthritis Rheum. 2017;69(5):1016–27
2Garris C, et al. J Med Econ. 2013;16(5):667–77
To cite this abstract in AMA style:
Lokhandwala T, Yue B, Coutinho A, Bell C. Within-Trial Cost Analysis of Flares from a Phase 3 Clinical Trial Evaluating Subcutaneous Belimumab for the Treatment of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/within-trial-cost-analysis-of-flares-from-a-phase-3-clinical-trial-evaluating-subcutaneous-belimumab-for-the-treatment-of-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/within-trial-cost-analysis-of-flares-from-a-phase-3-clinical-trial-evaluating-subcutaneous-belimumab-for-the-treatment-of-systemic-lupus-erythematosus/