Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Hepatitis B reactivation in the setting of rituximab use is a potentially fatal but preventable event. The rate of hepatitis B screening in patients receiving rituximab treatment is unknown. We used electronic health record (EHR) data to examine the proportion of patients who received adequate Hepatitis B screening prior to starting rituximab and also assessed patient and provider factors associated with inadequate screening.
Methods: Data derive from the EHR of a university health system, including diagnosis grouper codes, problem lists, medications, laboratory results, procedures codes, clinical encounter notes, and scanned documents. Data were extracted through back-end access to the EHR and supplemented via chart review by 2 physicians. We defined a cohort of patients who received rituximab between 6/1/2012 and 3/1/2016 (“index date”). We calculated the proportion of rituximab users who received inadequate screening for hepatitis B according to the Centers for Disease Control guidelines for detecting latent Hepatitis B infection (Hepatitis B surface antigen and Hepatitis B core antibody tests) prior to their first rituximab infusion during the study period. We used a relative risk regression model to identify independent predictors of inadequate testing. Variables included in the models were decided a priori and based on previously reported predictors of risk for Hepatitis B that may have influenced a provider’s decision to screen.
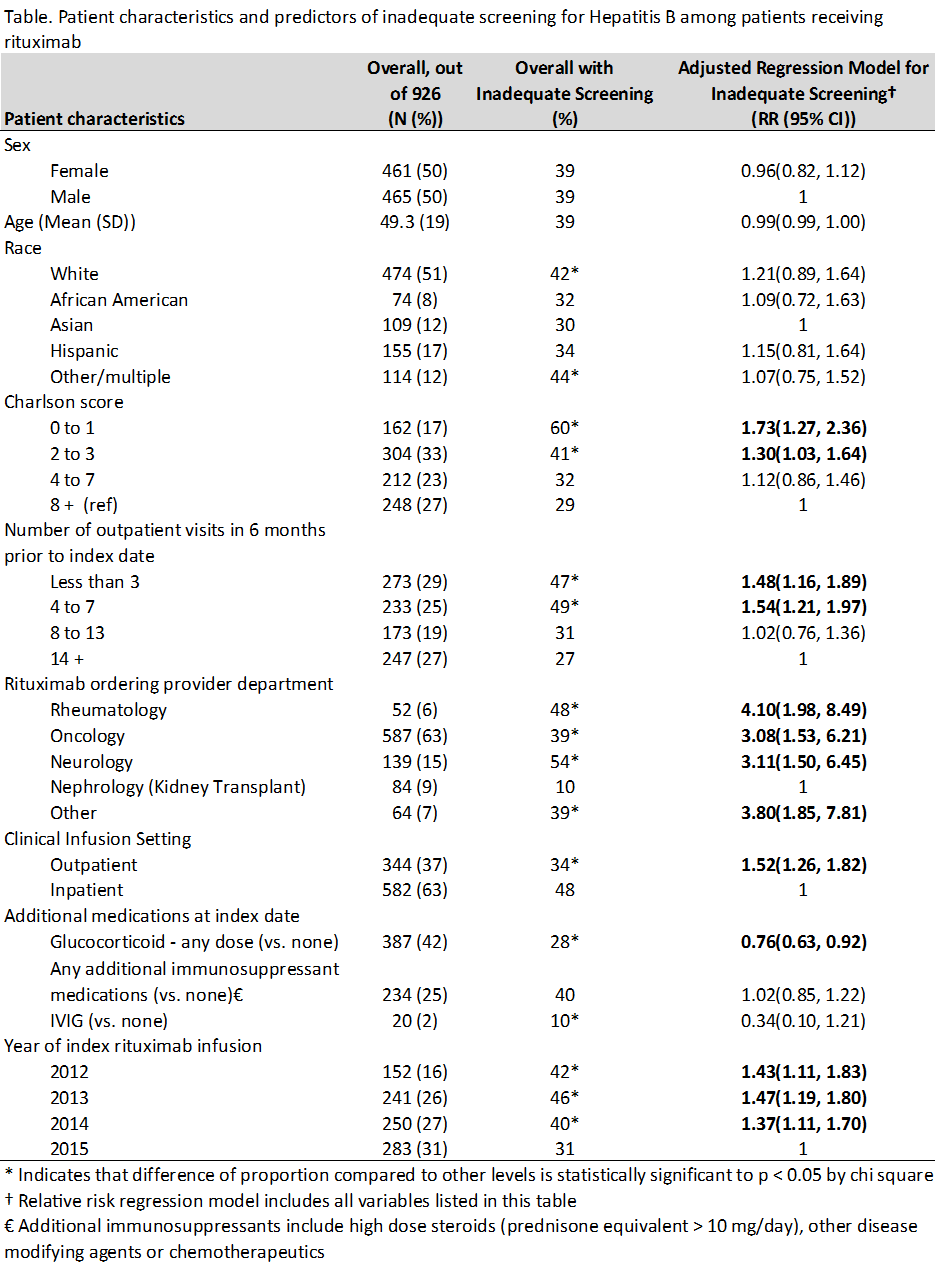
Results: 926 patients received rituximab during the study period. Mean age was 49 years; 50% were women; 51% were white. Most received rituximab for treatment of lymphoma (52%), kidney transplant rejection (10%), or multiple sclerosis (10%)– only 6% of orders were written by rheumatologists, for diagnoses such as vasculitis (4%) and rheumatoid arthritis (2%). Additional patient characteristics are shown in the Table. 565 (61%) patients had adequate screening for Hepatitis B; 214 (23%) had no Hepatitis B test documented at all. Nephrologists treating renal transplant rejection were most likely to have adequate screening (90%). Multivariate regression showed that the strongest predictor of inadequate screening was department of the ordering provider (see Table).
Conclusion: We found wide variations in Hepatitis B screening practices among patients receiving rituximab, resulting in unnecessary risks to patient safety. Strategies including provider education, use of checklists, and clinical decision support within the EHR should be developed to improve rates of screening in this high-risk patient population.
To cite this abstract in AMA style:
Schmajuk G, Tonner C, Trupin L, Li J, Yazdany J. Wide Variations in Hepatitis B Screening Practices for Patients Receiving Rituximab [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/wide-variations-in-hepatitis-b-screening-practices-for-patients-receiving-rituximab/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/wide-variations-in-hepatitis-b-screening-practices-for-patients-receiving-rituximab/