Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with axial spondyloarthritis (axSpA) and high disease activity (typically defined as Ankylosing Spondylitis Disease Activity Score [ASDAS]≥2.1), it is recommended to adapt treatment. However, this recommendation is not always followed in practice. The ASDAS was developed for research, and it is unknown how it performs in daily practice. Possibly, the cut-off of 2.1 as currently endorsed is too strict in this setting. Our objective was to investigate which ASDAS cut-off values correspond best with treatment intensification (TI) in practice.
Methods: Data were used from a prospective multicentre Dutch registry for patients with SpA (SpA-Net). Patients with axSpA and ≥1 ASDAS measurement in 2016-2022 were included. TI was defined as 1) higher dose or frequency of the same drug, 2) switch to another drug or 3) addition of a new drug to the current treatment regime; due to inefficacy. Only anti-inflammatory drugs (NSAIDs, conventional synthetic/biologic/targeted synthetic DMARDs [cs/b/tsDMARDs], corticosteroids) were considered. Single patients could contribute multiple TI events. Receiver operating characteristic (ROC) curve analyses were conducted to estimate the ability of ASDAS to discriminate between TI/non-TI (Area Under the Curve [AUC]), and identify the ASDAS cut-off that discriminates best in this real-world population, with corresponding sensitivity and specificity. Analyses were conducted with (1) all observations and (2) the first observation per patient per calendar year.
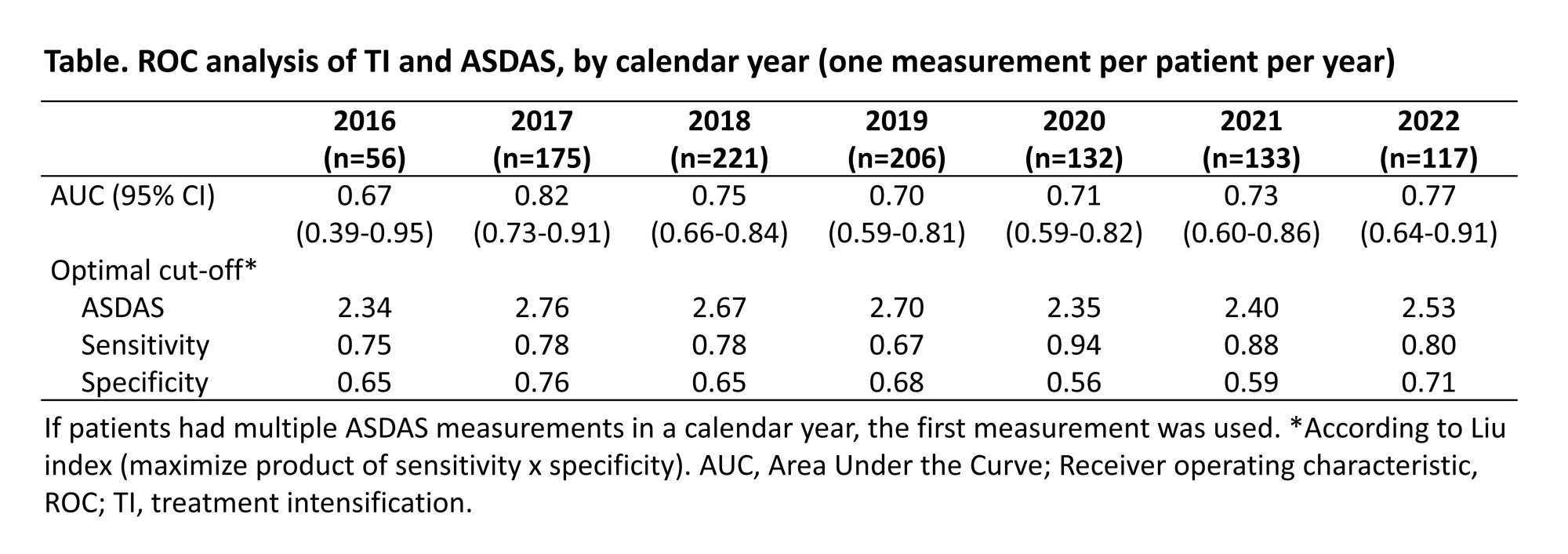
Results: In total, 328 patients with 2,010 ASDAS measurements were included. Median follow-up was 2.5 (IQR 1.0-4.3) years. Mean age was 50.2 (SD 14.0) years, 149 (45.4%) were female, and mean ASDAS was 2.3 (SD 1.0). Approximately two-thirds of patients (211/328, 64.3%) were on biological/targeted synthetic DMARDs (b/tsDMARDs) at some point during follow-up. TI was applied after 218/2,010 ASDAS measurements (10.8%), and mean ASDAS was higher at TI timepoints than at non-TI timepoints (3.1 [SD 1.0] versus 2.3 [SD 1.0]). When all ASDAS measurements were included for analysis, the AUC was 0.72 (95%CI 0.68-0.75) with an optimal ASDAS cut-off of 2.7 (sensitivity 68%, specificity 66%). Results were similar when only one measurement was used per patient and calendar year (1,069 ASDAS measurements; AUC 0.75, ASDAS cut-off 2.7). Over the years, the ASDAS cut-off was consistently higher than 2.1, however with a decreasing trend (Table).
Conclusion: In daily practice, TI is associated with a higher ASDAS cut-off value than the recommended one (≥2.1). Possibly, rheumatologists believe the recommended cut-off to be too stringent or consider other factors than disease activity when making treatment decisions. Regardless, the cut-off seems to be gradually decreasing, suggesting increased uptake of axSpA management recommendations.
To cite this abstract in AMA style:
Webers C, Nezam El-Din R, Been M, Vonkeman H, van Tubergen A. Which ASDAS Cut-Off Corresponds Best to Treatment Intensification in Patients with Axial Spondyloarthritis in Daily Practice? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/which-asdas-cut-off-corresponds-best-to-treatment-intensification-in-patients-with-axial-spondyloarthritis-in-daily-practice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/which-asdas-cut-off-corresponds-best-to-treatment-intensification-in-patients-with-axial-spondyloarthritis-in-daily-practice/