Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Recent recommendations have suggested higher starting doses of methotrexate, i.e. 15 mg/week (3E initiative) in rheumatoid arthritis. However, studies comparing conventional (7.5mg) and newer (15mg) starting dose are limited. Unclear if any difference in control of disease activity or adverse effects with these starting doses.
Methods: This randomized controlled trial included 100 rheumatoid arthritis patients (fulfilling 1987 ACR), having active disease (‘Disease activity score 28 joints 3 variables’ DAS28-3v³5.1) and not on methotrexate ³ 3 months. Using random number tables, patients were allocated (concealed by envelopes) to two groups – starting methotrexate at 7.5mg or 15 mg/week. Methotrexate escalated by 2.5 mg every 2 weeks; similar folic acid dose (10 mg/week). Patients assessed 4 weekly (blinded assessor) for disease activity (DAS28-3v). In addition, minor adverse effects (symptoms like nausea etc) determined using a performa and major adverse effects determined using blood tests (cytopenias– WBC<4000, Platelet<1lac or transaminitis– ALT or AST>80) or CXR (PA) if required. Analysis by intention to treat; difference in disease activity, adverse effects compared using students t and chi-square test respectively (or Fishers exact). (Trial Reg# NCT01404429)
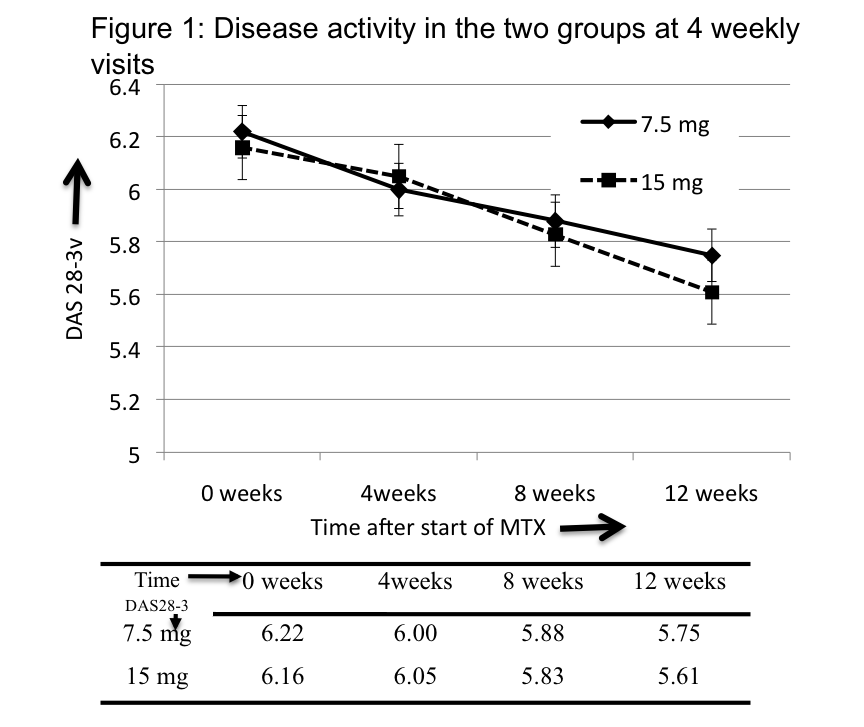
Results: Patients in the two groups – 7.5 mg and 15 mg had similar age (44.5±10.3, 42.8±11.2 yrs, p=0.4), gender (F:M=36:11, 42:11, p=0.4), disease duration (4.8±4.8, 4.7±4.5 yrs, 0.9), disease activity (DAS28-3v=6.2±0.7, 6.2±0.8, p=0.9) and HAQ (1.3,1.3,p=0.9). In the 7.5 and 15 mg groups, 38 and 46 patients completed study, reaching final methotrexate dose of 19.3±1.8 mg and 24.3±2.0 mg per week respectively. Significant reduction in disease activity in both groups at 12 weeks (p<0.001), however, there was no intergroup difference at 4, 8 or 12 weeks (Figure 1), nor in HAQ at 12 weeks (1.1,0.9,p=0.4). No difference in 7.5 and 15mg groups in episodes of cytopenias (1,2, p=0.9) or transaminitis (6,7, p=0.8) or pneumonitis (0,0). However, minor adverse effects lower in 7.5mg compared to 15mg group (Relative Risk=0.58, 95% CI:0.36-0.95). Common were nausea (more in 15mg), loss of appetite, fatigue and uneasiness. (Table 1)
Conclusion: Starting treatment with either 7.5 mg or 15 mg per week of methotrexate followed by similar fast escalation (5mg/month) is equivalent in control of disease activity at 12 weeks. Although, no difference in transaminitis or cytopenia, higher minor adverse effects, especially nausea in 15mg group.
Table 1: Frequency of minor adverse effects (symptoms) in the two groups
|
Minor adverse effect (Symptom)
|
7.5 mg group
|
15 mg group
|
P value
|
Relative Risk 7.5 versus 15 mg (95% confidence intervals)
|
|
Any |
31.9 |
54.7 |
0.02* |
0.58 (0.36-0.95) |
|
Nausea |
19.2 |
39.6 |
0.02* |
0.48(0.25, 0.94) |
|
Fatigue |
6.4 |
13.2 |
0.43 |
0.48(0.13, 1.76) |
|
Loss of appetite |
12.8 |
11.3 |
0.80 |
1.13(0.39, 3.26)
|
|
Diarrhea |
0.0 |
5.7 |
0.29 |
0.18 (0.01, 3.62) |
|
Uneasiness or dizziness |
17.0 |
13.2 |
0.59 |
1.29 (0.51, 3.28) |
* statistically significant
Disclosure:
V. Dhir,
None;
M. Singla,
None;
P. Goyal,
None;
V. Sagar,
None;
A. Sharma,
None;
S. K. Sharma,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-is-the-right-dose-to-start-methotrexate-7-5-or-15mg-in-rheumatoid-arthritis-a-randomized-controlled-trial/