Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Axial spondyloarthritis (axSpA) is a disease with a rather heterogeneous presentation that may be difficult to diagnose. Classification criteria, such as the ASAS criteria, developed and validated against the gold-standard ‘expert diagnosis’, exist, but may suffer from circularity because features deemed important by experts may have got a too prominent, and therefore biased, role. If classification criteria are used inappropriately to confirm a diagnosis, overdiagnosis may be an unwarranted consequence. We aimed to gain an unbiased insight into the concept of axSpA, by circumventing expert opinion and investigating its ‘latent constructs’: We examined the SpA-features’ mutual statistical coherence, established the unbiased ‘Gestalt’ of SpA, and evaluated how the ASAS axSpA criteria capture these ‘latent constructs’.
Methods: Two independent cohorts of patients (pts) with early onset chronic back pain (SPACE cohort) and inflammatory back pain (IBP) (DESIR cohort) were included. Latent class analysis (LCA) (different than a cluster analysis) was used to estimate the latent (i.e. unobserved) ‘Gestalt’ of axSpA by modelling the covariance of the observed SpA features (without ‘a priori’ assumptions on their ‘weights’). The selected best LCA model splits axSpA into a number of (clinically meaningful) classes with best data fit. Each class was labelled by us and named according to most prominent features. The latent axSpA classes were then used as ‘gold-standard’ against which the ASAS axSpA, pSpA (ignoring IBP) and both (SpA criteria) were tested. Finally, 5-year follow-up data from DESIR were used to perform a latent transition analysis (LTA) in order to examine if patients change classes over 5-year time.
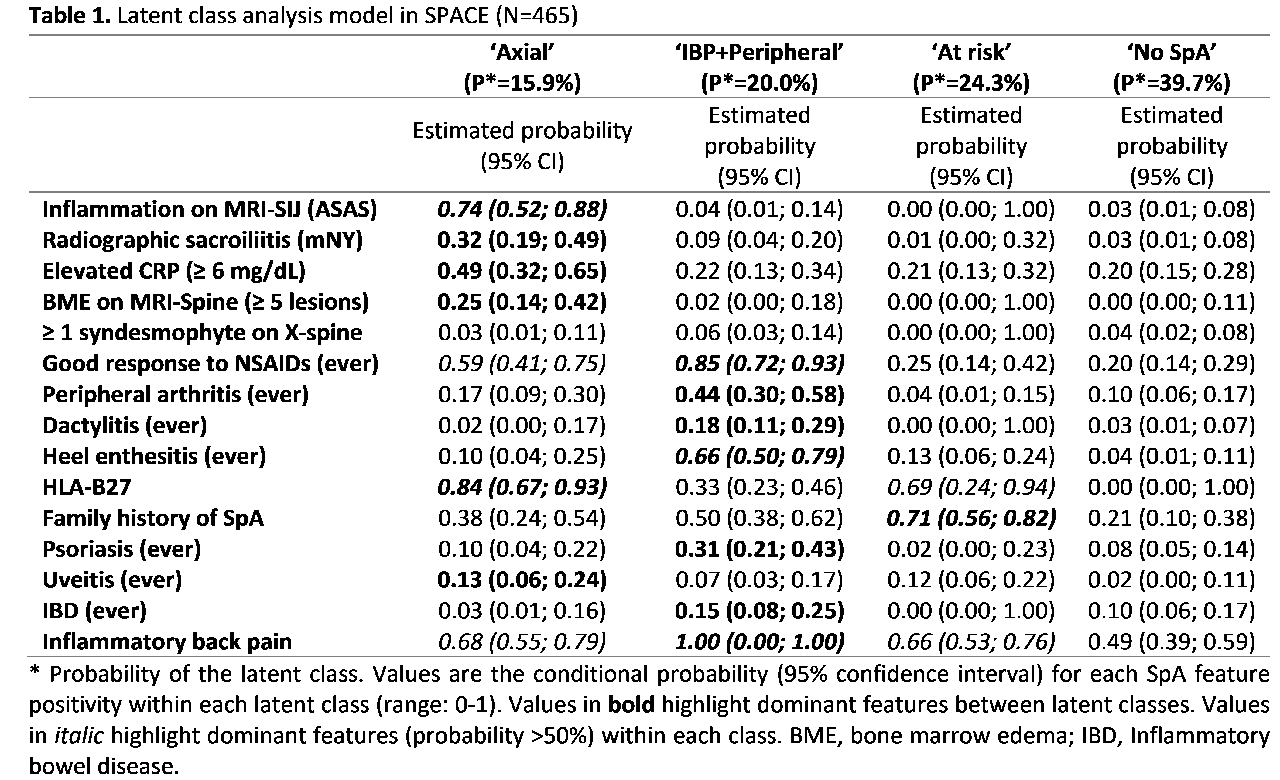
Results: In total, data of 465 (SPACE) and 576 (DESIR) pts were analyzed. SPACE yielded 4 latent classes (Table 1). The ‘Axial’ class characterized by highest likelihood on abnormal imaging and HLA-B27-positivity; the ‘IBP+Peripheral’ class had 100% likelihood of IBP in association with peripheral signs. The ‘At risk’ class is anchored on a positive family history and HLA-B27 positivity in association with IBP; and the ‘No SpA’ class had very low likelihoods for all SpA-features. The analysis in DESIR (without ‘no-SpA’ pts) yielded identical latent classes (‘Axial’:19%; ‘IBP+Peripheral’:27% and ‘At risk’:55%) (Table 2). The ASAS axSpA criteria, tested in SPACE (‘No SpA’ absent in DESIR), captured 67% of patients in the ‘Axial’ and ‘IBP+Peripheral’ classes (‘latent gold-standard’), but sensitivity was better (87%) if axSpA and pSpA criteria were combined. Of note, the axSpA criteria captured only 4% of the pts from the ‘No SpA’ class. The LTA suggests that transition between classes over time was unlikely. ‘Axial’ and ‘IBP+Peripheral’ patients did not switch and only 11% of ‘At risk’ pts had switched to ‘IBP+Peripheral’ after 5 years.
Conclusion: The ‘Gestalt’ of axial spondyloarthritis comprises three distinguishable clinical entities (‘pure axial SpA’, ‘axial SpA with peripheral signs, and ‘axial SpA at risk’). Patients keep their clinical entity over 5 years and transition is very rare. The ‘Axial’ and ‘IBP+Peripheral’ entities are best captured by combining the ASAS axSpA and pSpA criteria.
To cite this abstract in AMA style:
Sepriano A, Ramiro S, van der Heijde D, Hoonhout P, Moltó A, Saraux A, Dougados M, Landewé R. What Is Axial Spondyloarthritis? A Latent Class and Transition Analysis in the SPACE and DESIR Cohorts [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/what-is-axial-spondyloarthritis-a-latent-class-and-transition-analysis-in-the-space-and-desir-cohorts/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-is-axial-spondyloarthritis-a-latent-class-and-transition-analysis-in-the-space-and-desir-cohorts/