Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis is broadly characterized by clinical domains such as enthesitis, dactylitis, nail manifestations, and psoriasis. How these clinical domains influence the response to MTX and/or TNF inhibitor therapy remains unclear. Here we report the findings using data from the 48-week, SEAM-PsA trial.
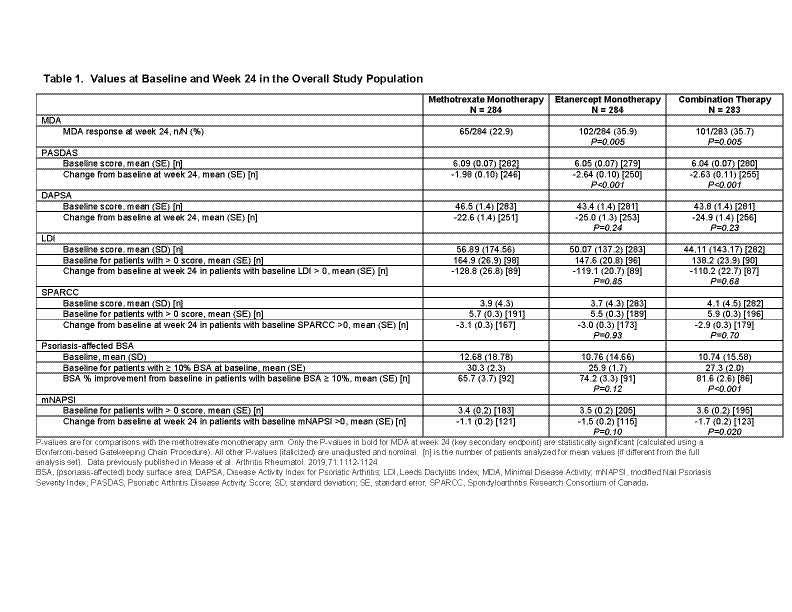
Methods: In the SEAM-PsA trial, MTX and biologic-naïve adult patients with active PsA were randomized to three treatment arms; MTX (20 mg; N=284); ETN (50 mg; N=284); or Combo (ETN 50 mg + MTX 20 mg; N=283), given weekly. Clinical outcomes (week 24) were measured as a percentage of patients achieving MDA, a change from baseline in PASDAS, and DAPSA scores. Patient subsets were based on clinical domains such as enthesitis (SPARCC), dactylitis (LDI), nail manifestations (mNAPSI) or psoriasis (BSA). Analyses comparing the ETN-containing arms with the MTX monotherapy arm used an ANCOVA model and was adjusted for baseline BMI status, prior non-biologic DMARD use, and a disease manifestation baseline status interaction term. P-values were unadjusted for multiplicity.
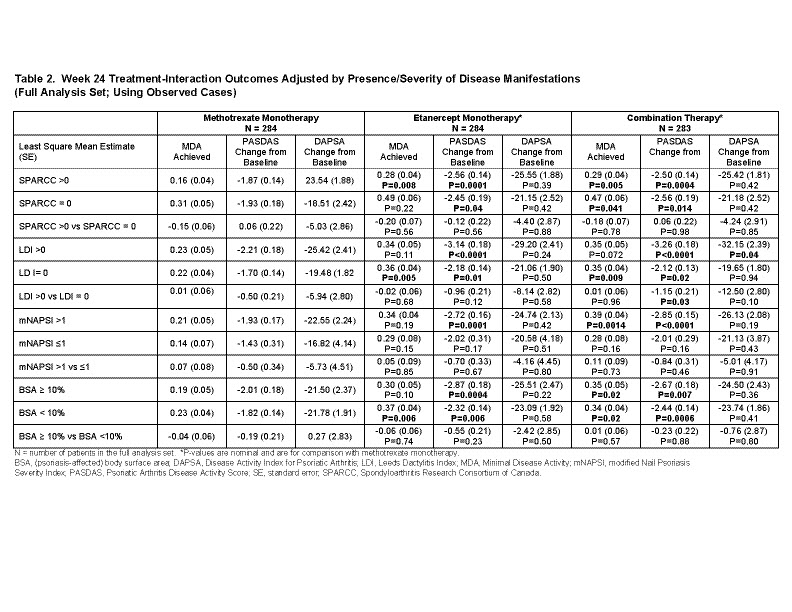
Results: The 3 treatment arms had similar baseline values (Table 1). Among patients with enthesitis, without dactylitis, and BSA< 10%, those patients receiving ETN monotherapy were more likely to achieve MDA compared with MTX monotherapy. The greater change in PASDAS scores observed in ETN monotherapy compared with MTX monotherapy were not influenced by the presence or absence of enthesitis, dactylitis or BSA>10% (Table 2). Changes in DAPSA scores were similar across all clinical domains and treatment arms except for those patients with dactylitis in the Combo arm (Table 2).
Conclusion: For clinical domains that demonstrated differences between treatment arms, ETN arms presented better clinical outcomes when compared with MTX monotherapy. In addition, MDA and PASDAS reported consistent differences between treatment arms and are more sensitive at detecting therapy-mediated changes between clinical domains, whereas no differences were detected with DAPSA, except for dactylitis.
To cite this abstract in AMA style:
Helliwell P, Mease P, Kavanaugh A, Coates L, Ogdie A, Deodhar A, Strand V, Maksabedian E, Kricorian G, Liu L, Collier D, Gladman D. What Influence Do Clinical Domains Other Than Arthritis Have on Composite Clinical Outcomes in Psoriatic Arthritis?: Comparison of Treatment Effects in the SEAM-PsA Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/what-influence-do-clinical-domains-other-than-arthritis-have-on-composite-clinical-outcomes-in-psoriatic-arthritis-comparison-of-treatment-effects-in-the-seam-psa-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-influence-do-clinical-domains-other-than-arthritis-have-on-composite-clinical-outcomes-in-psoriatic-arthritis-comparison-of-treatment-effects-in-the-seam-psa-trial/