Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
To characterize which factors are associated with RA
patients’ preferences for biologic administration: sub-cutaneous or
intravenous.
Methods:
Using a large US observational cohort, the National Data
Bank for Rheumatic Diseases (NDB), we characterized RA patients who were on a
biologic from Jan 2006 onwards, one year after Medicare changed reimbursement policies
to cover more biologics than infliximab. Only the patient’s first biologic or
second (if already on their first biologic) were considered. Patients
were assigned into 3 groups based on biologic administration: intravenous (IV),
sub-cutaneous (SC), or “both” if they had taken IV and SC biologics in the same 6-month
period. All patients enrolled
via FDA-mandated drug safety registries were excluded, as well as those who
had not had any change on biologic use after enrollment.
One–way Anova and chi-squared tests were used when applicable, with
5% significance level. Polytomous logistic
regression models were used, with SC as reference, controlling for confounders,
and results were presented as relative risk ratios (RRR). All explanatory variables were 6-month
lagged, including: socio-economic status, marital status, markers of clinical
status such as HAQ, Patient Activity Score, SF-36 physical component summary
score, and dependency on others for help.
Results:
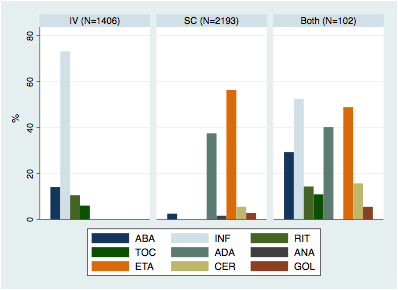
From 4,318 eligible RA patients, 1,609 (37.3%) were taking an
IV biologic, 2,562 (59.3%) were on a SC biologic and 147 patients (3.4%) were
on both (Figure 1). Patients in the IV group tended to be older, have longer RA
duration, more likely on Medicare, and depended less frequently on others for
help (Table
1). They also tended to have greater disability and worse disease activity (IV
vs. SC RRR for HAQ, 1.21 [1.09-1.34]; both vs. SC, 1.61 [1.23; 2.12]).
Patients
who switched between IV and SC were more likely to have uncontrolled disease
activity. Most patients on biologics (70.8%) did not switch between classes, and the most common order of switching was from SC
to IV (62.3%). which is consistent with the premise that patients/physicians
may have preferences for one format over the other.
Conclusion:
Our results
consistently characterized patients on IV biologics as more likely to choose
and/or require the professional help that comes with IV administration. As most
patients did not switch, this may indicate overall preferences by the patient
and/or physician.
Figure 1. Biologic
distribution by form of administration (%).
Table 1. Multivariate
polytomous logistic regression of IV vs. SC patients
(N=4,002)
|
Variables
|
RRR
|
95% Conf. Interval
|
P-value
|
|
|
How often do you depend on other for help? |
||||
|
None (referent)
|
1 |
|
|
|
|
A little of the time
|
1.26 |
1.04 |
1.52 |
0.02 |
|
Some of the time
|
1.16 |
0.94 |
1.42 |
0.16 |
|
Most of the time
|
1.34 |
0.98 |
1.82 |
0.06 |
|
All of the time
|
1.70 |
0.79 |
3.62 |
0.17 |
|
Age (yrs)
|
1.03 |
1.01 |
1.03 |
0.00 |
|
RA duration (yrs)
|
0.99 |
0.99 |
1.00 |
0.04 |
|
Comorbidity index |
1.01 |
0.97 |
1.06 |
0.55 |
|
Total income (US$1000) |
1.00 |
1.00 |
1.00 |
0.17 |
|
Education (yrs) |
1.01 |
0.98 |
1.05 |
0.42 |
|
Male sex |
0.97 |
0.81 |
1.16 |
0.75 |
|
Employed |
0.94 |
0.79 |
1.13 |
0.51 |
|
Medical Insurance |
||||
|
Private (referent)
|
|
|
|
|
|
Medicare*
|
1.91 |
1.56 |
2.33 |
0.00 |
|
Medicaid
|
1.18 |
0.87 |
1.62 |
0.29 |
|
None
|
0.65 |
0.36 |
1.17 |
0.15 |
|
Constant |
0.13 |
0.07 |
0.25 |
0.00 |
To cite this abstract in AMA style:
Michaud K, Pedro S, Peterson S. What Factors Are Associated with Starting an Intravenous Vs. Sub-Cutaneous Biologic in Patients with RA? [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/what-factors-are-associated-with-starting-an-intravenous-vs-sub-cutaneous-biologic-in-patients-with-ra/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-factors-are-associated-with-starting-an-intravenous-vs-sub-cutaneous-biologic-in-patients-with-ra/