Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: BICLA is a validated composite global measure of SLE disease activity that incorporates BILAG, an instrument that distinguishes between partial and complete improvement. BICLA was an endpoint in the phase 3 TULIP-1 and TULIP-2 trials of anifrolumab.1,2 This study investigated the relationships between BICLA response and SLE clinical and laboratory assessments in TULIP-1 and -2, irrespective of treatment assignment.
Methods: This was a post hoc analysis of pooled data from the 52-week (wk), double-blind TULIP-1 and -2 trials. Patients with moderately to severely active SLE, despite standard of care, were randomized to receive anifrolumab (150 or 300 mg IV Q4W) or placebo for 48 wks. BICLA responses were defined by the following: reduction of all baseline BILAG-2004 A and B domain scores to B/C/D and C/D, respectively, and no worsening in any organ system; no worsening of SLEDAI-2K score; and no worsening ≥0.3 points in Physician’s Global Assessment (range 0–3).3 Attempts to taper oral corticosteroids (OCS) to ≤7.5 mg/day between Wks 8 and 40 were required for patients receiving OCS ≥10 mg/day at baseline. Sustained OCS dosage reduction was defined as OCS dosage ≤7.5 mg/day achieved by Wk 40 and sustained through Wk 52.
Results: Baseline characteristics were generally similar between BICLA responders (n=318) and nonresponders (n=501). Overall, improved outcomes were observed in BICLA responders vs nonresponders, including numerically greater improvements in SLEDAI-2K (–7.4 [SD: 3.64] vs –4.2 [SD: 4.28]) from baseline to Wk 52 (Table 1). Greater mean daily OCS dosage reduction was observed in BICLA responders vs nonresponders (–5.41 [SD: 6.84] vs –1.67 [SD: 8.08] mg/day) from baseline to Wk 52, and sustained OCS dosage reduction was achieved by more BICLA responders vs nonresponders (79.2% vs 19.1%). A ≥50% reduction in Cutaneous Lupus Erythematosus Disease Area and Severity Index activity (CLASI-A) score, for patients with baseline score ≥10, was achieved by more BICLA responders vs nonresponders (92.0% vs 23.2%) at Wk 52. Greater reductions of mean anti-dsDNA antibody levels were observed in BICLA responders vs nonresponders (–46.1 [SD: 335.69] vs 15.8 [SD: 450.92] U/mL) from baseline to Wk 52; numeric improvements were also observed for complement C3 (Table 2). More patients who were BICLA responders vs nonresponders reported improved patient-reported outcomes, with greater improvements in the Functional Assessment of Chronic Illness Therapy-Fatigue of >3 points (55.6% vs 15.7%) and the Short Form 36 Health Survey physical component summary of >3.4 (57.9% vs 12.8%) (Table 3).
Conclusion: BICLA response was associated with clinical benefit in multiple SLE measures, including SLEDAI-2K, CLASI-A, OCS dosage reduction, and patient-reported outcomes. These data uphold the value of BICLA as an endpoint in SLE trials and also expand its benefit to translating trial data to metrics that are clinically meaningful in everyday practice.
References
- Morand EF. N Engl J Med. 2020;382:211–21.
- Furie RA. Lancet Rheumatol. 2019;1:e208–19.
- Wallace DJ. Ann Rheum Dis. 2014;73:183–90.
Writing assistance by Rosie Butler, PhD (JK Associates Inc., a Fishawack Health Company).
This study was sponsored by AstraZeneca.
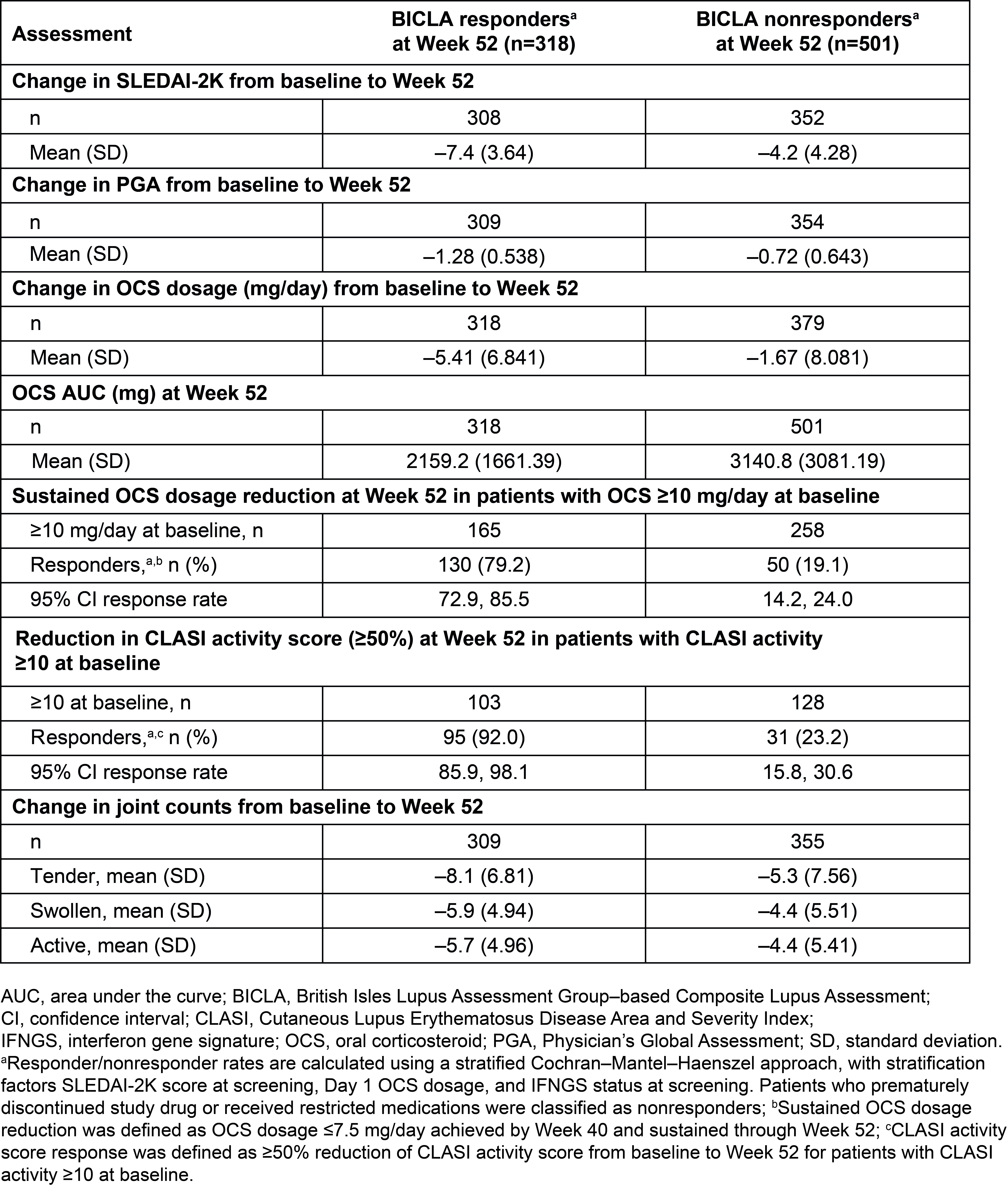 Table 1. SLE Assessments in BICLA Responders vs Nonresponders in Pooled Data From the Phase 3 TULIP-1 and TULIP-2 Trials
Table 1. SLE Assessments in BICLA Responders vs Nonresponders in Pooled Data From the Phase 3 TULIP-1 and TULIP-2 Trials
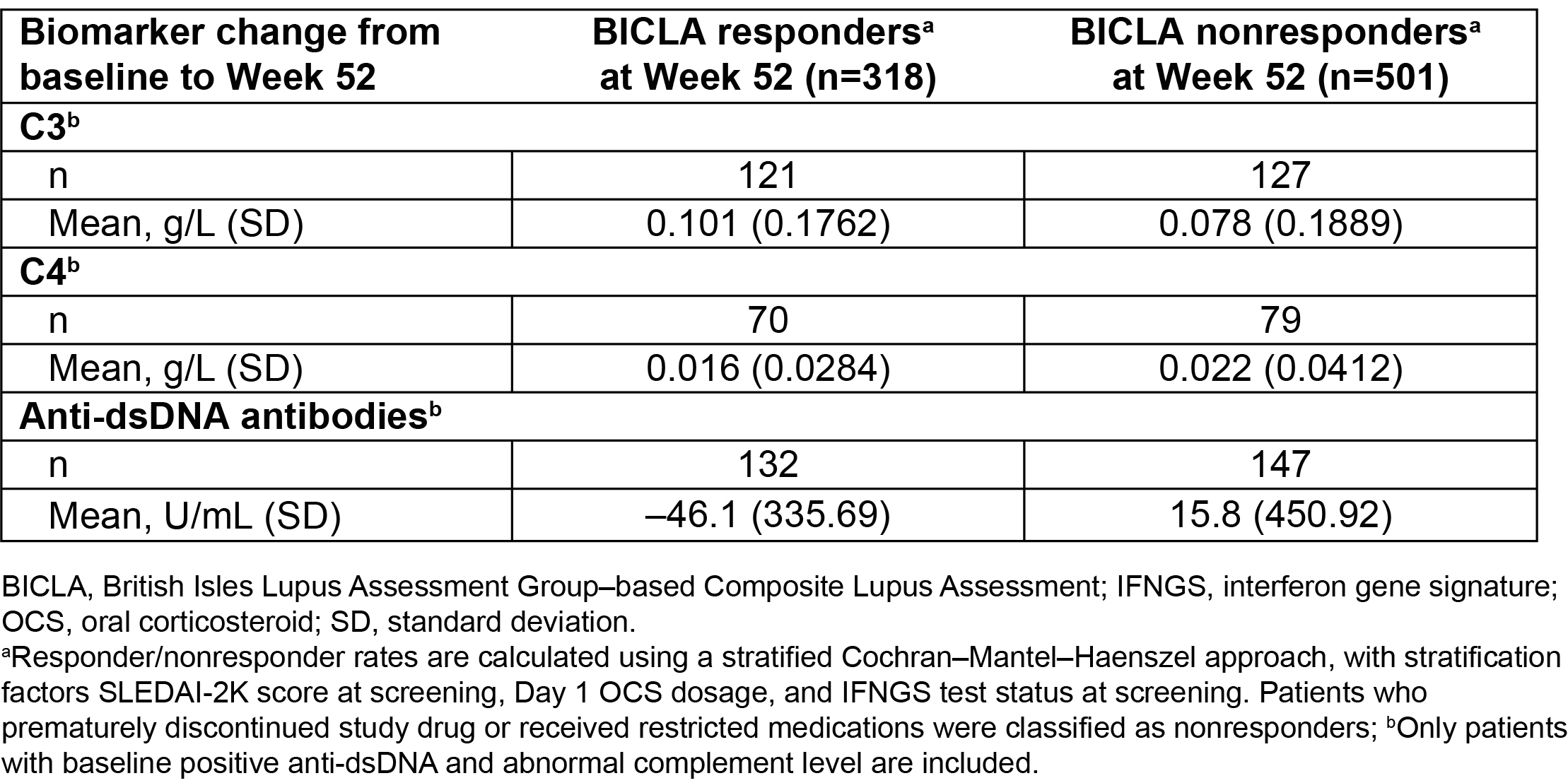 Table 2. Change in Biomarker Levels From Baseline to Week 52 in BICLA Responders vs Nonresponders in Pooled Data From the TULIP Trials
Table 2. Change in Biomarker Levels From Baseline to Week 52 in BICLA Responders vs Nonresponders in Pooled Data From the TULIP Trials
 Table 3. Improvement in Patient-Reported Outcome Assessments From Baseline to Week 52 in BICLA Responders vs Nonresponders in Pooled Data From the TULIP Trials
Table 3. Improvement in Patient-Reported Outcome Assessments From Baseline to Week 52 in BICLA Responders vs Nonresponders in Pooled Data From the TULIP Trials
To cite this abstract in AMA style:
Furie R, Morand E, Bruce I, Isenberg D, van Vollenhoven R, Abreu G, Pineda L, Tummala R. What Does It Mean to Be a BICLA (BILAG-Based Composite Lupus Assessment) Responder? Post Hoc Analysis of the Phase 3 TULIP-1 and TULIP-2 Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/what-does-it-mean-to-be-a-bicla-bilag-based-composite-lupus-assessment-responder-post-hoc-analysis-of-the-phase-3-tulip-1-and-tulip-2-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-does-it-mean-to-be-a-bicla-bilag-based-composite-lupus-assessment-responder-post-hoc-analysis-of-the-phase-3-tulip-1-and-tulip-2-trials/
