Session Information
Session Type: Abstract Submissions (ACR)
Conclusion: Time to onset and magnitude of ACR 20 and HAQ-DI responses and DAS28 improvements were similar for pts treated with SC abatacept with or without IV loading in patients with RA and an inadequate response to MTX. Previous pharmacokinetic data show that in the absence of IV loading, target therapeutic concentrations are achieved in the majority of pts by Week 2 of SC abatacept treatment.3 The findings from this post-hoc analysis suggest that SC abatacept can be given effectively without an IV abatacept loading dose. 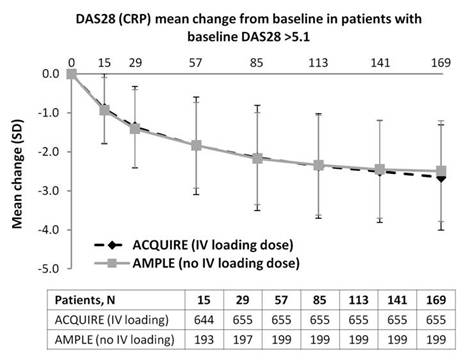
Disclosure:
M. Schiff,
Bristol-Myers Squibb,
5,
Abbott Immunology Pharmaceuticals,
8;
R. Alten,
ABBOTT, BMS, GSK,NOVARTIS, PFIZER, UCB,
2;
M. Weinblatt,
Bristol-Myers Squibb,
2,
Bristol-Myers Squibb,
5;
P. Nash,
Bristol-Myers Squibb,
2,
Bristol-Myers Squibb,
5,
Bristol-Myers Squibb,
8;
R. Fleischmann,
Genentech Inc., Roche, Abbott, Amgen, UCB, Pfizer, BMS, Lilly, Sanofi Aventis, Lexicon, MSD, Novartis, BiogenIdec, Astellas, Astra-Zeneca, Jansen,
2,
Roche, Abbott, Amgen, UCB, Pfizer, BMS, Lilly, Sanofi Aventis, Lexicon, Novartis, Astellas, Astra-Zeneca, Jansen, HGS,
5;
P. Durez,
BMS – Less than US$2000 ,
8;
J. Kaine,
Bristol-Myers Squibb,
5,
Bristol-Myers Squibb,
8;
I. Delaet,
Bristol-Myers Squibb,
1,
Bristol-Myers Squibb,
3;
S. Kelly,
Bristol-Myers Squibb,
1,
Bristol-Myers Squibb,
3;
M. Maldonado,
Bristol-Myers Squibb,
1,
Bristol-Myers Squibb,
3;
S. Patel,
Bristol-Myers Squibb,
3;
M. C. Genovese,
Bristol-Myers Squibb,
2,
Bristol-Myers Squibb,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/weekly-subcutaneous-abatacept-confers-comparable-onset-of-treatment-response-and-magnitude-of-efficacy-improvement-over-6-months-when-administered-with-or-without-an-intravenous-abatacept-loading-dose/
