Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Patients with psoriasis often develop psoriatic arthritis (PsA), a chronic systemic inflammatory disease with a severe clinical burden that impairs quality of life. In recent years, interleukin-17 (IL-17) has gained attention as a potential target for the treatment of PsA. The study aimed to evaluate the efficacy and safety of vunakizumab (SHR-1314), a humanized anti-IL-17A monoclonal antibody, at different doses in adult patients with active PsA.
Methods: This was a multicenter, randomized, double-blind, phase 2 clinical trial (ClinicalTrials.gov, NCT05055934). Eligible patients were aged 18 to 75 years; had a confirmed diagnosis of PsA per the Classification of Psoriatic Arthritis criteria; had symptoms for at least 6 months before screening; had active disease at baseline (a tender joint count of at least 3 [of 68] and a swollen joint count of at least 3 [of 66]); and had at least one active psoriatic lesion or documented history of psoriasis. Patients were randomized (1:1:1) to receive subcutaneous vunakizumab at 240 mg, vunakizumab at 120 mg, or placebo at weeks 0, 2, 4, and 8. At week 12, patients originally assigned to vunakizumab continued to receive vunakizumab (Q4W) and patients assigned to placebo were switched to vunakizumab (1:1 re-randomized to 240 mg or 120 mg dose, administered at weeks 12, 14, 16, and 20). The primary endpoint was American College of Rheumatology 20% improvement (ACR20) response rate at week 12. Secondary endpoints included ACR50, ACR70, Disease Activity Score Based on C-Reactive Protein (DAS28-CRP), Health Assessment Questionnaire-Disability Index (HAQ-DI), and Psoriasis Area and Severity Index (PASI; in patients with body surface area affected by psoriasis ≥3%) at week 12.
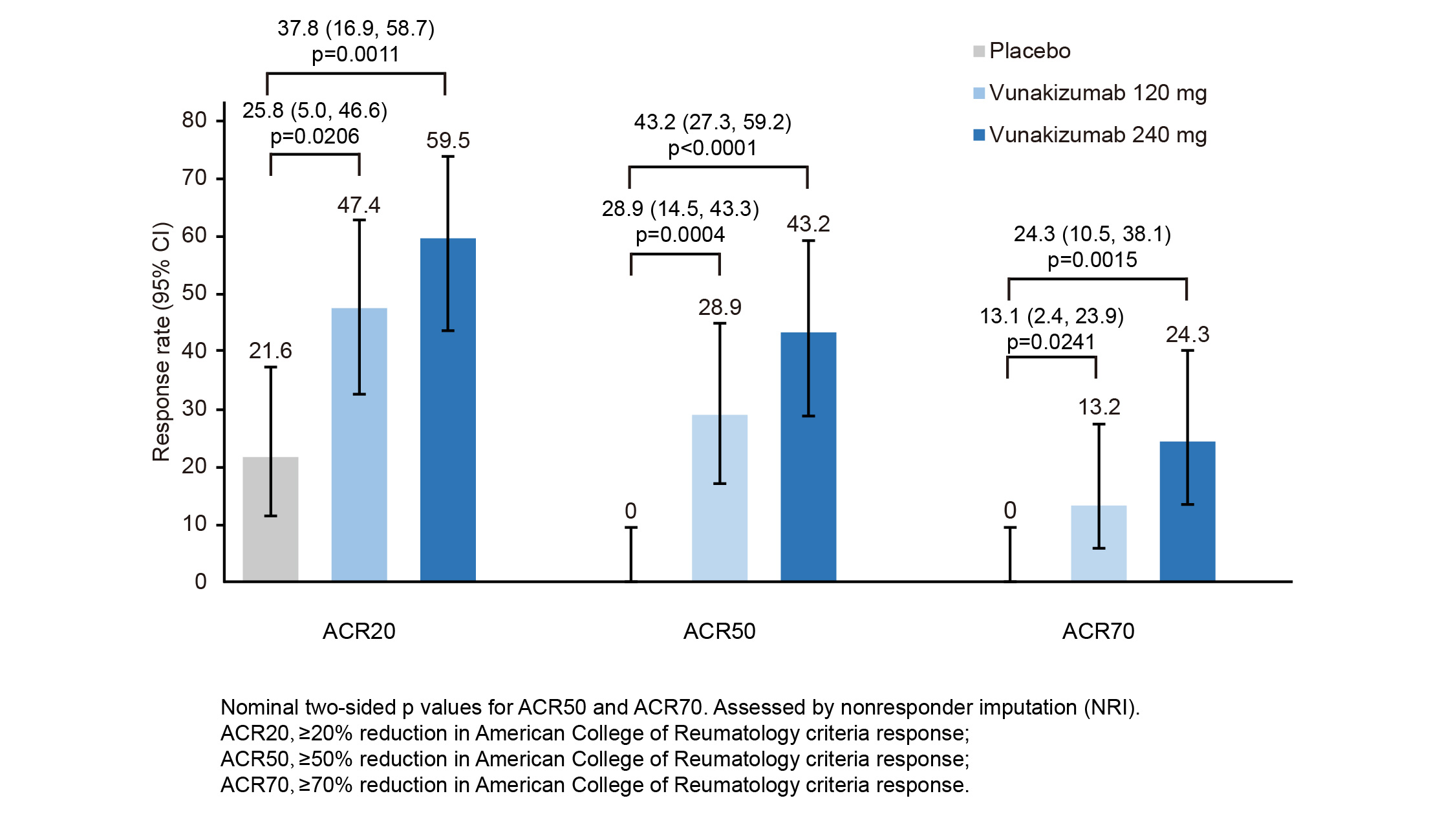
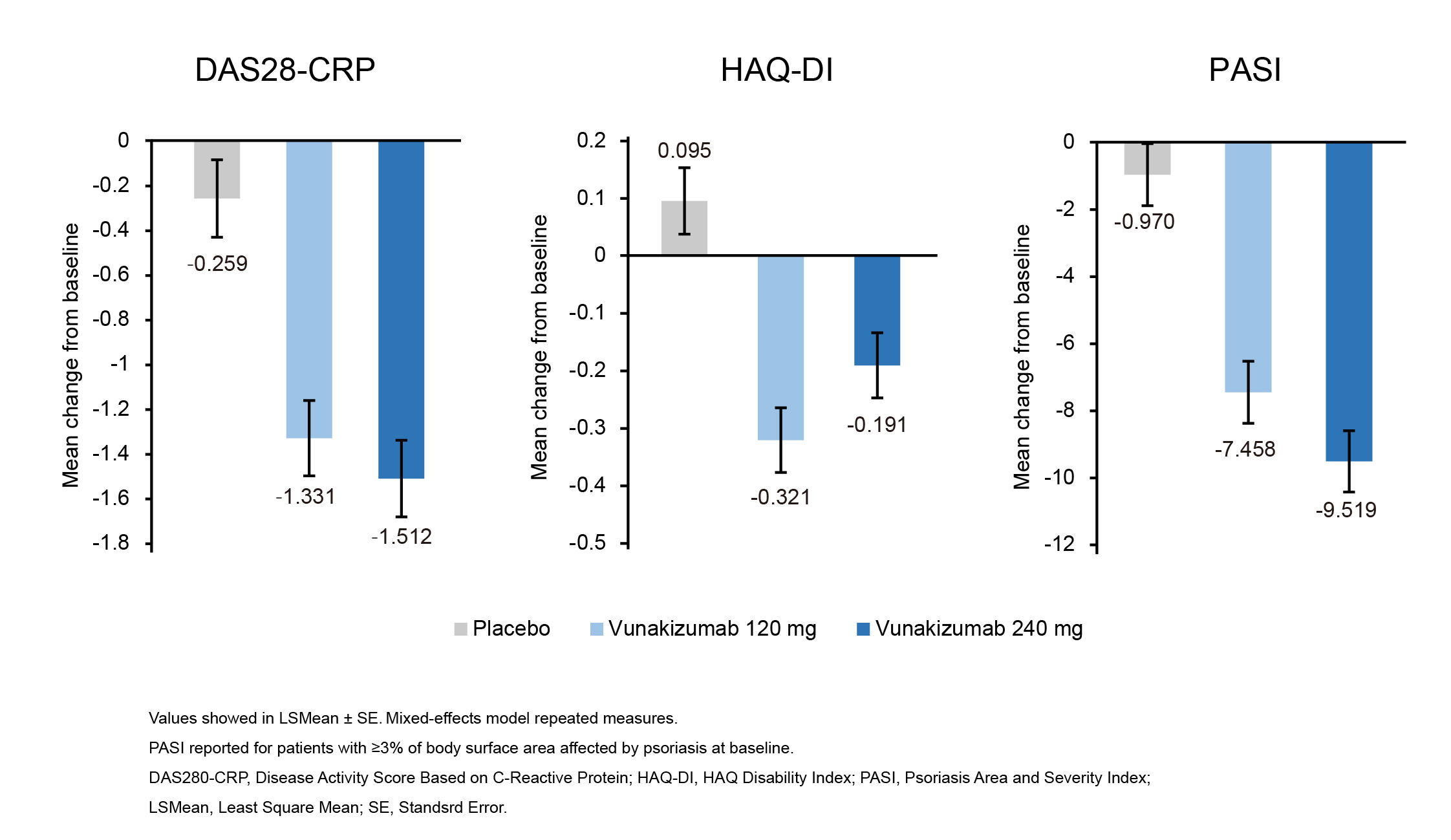
Results: In total, 112 patients were randomized (37 in vunakizumab 240 mg group, 38 in vunakizumab120 mg group, and 37 in placebo group). Demographics and baseline characteristics were balanced across groups. A higher percentage of patients treated with vunakizumab at 240 mg (59.5%) or 120 mg (47.4%) achieved ACR20 at week 12 compared with placebo (21.6%; two-sided p = 0.0011 and p = 0.0206, respectively; Figure 1). Also, more percentage of vunakizumab-treated patients achieved ACR50 and ACR70 at week 12 (Figure 1). Furthermore, patients treated with vunakizumab demonstrated a greater least square mean change in DAS28-CRP, HAQ-DI, and PASI from baseline to week 12 than those treated with placebo, across both doses (Figure 2). Throughout the 12-week treatment period, the incidences of treatment-emergent adverse events (TEAEs; 64.9%, 73.7% vs 67.6%) were similar for patients receiving vunakizumab (240 mg or 120 mg) or placebo, and the most common TEAE was upper respiratory tract infection in vunakizumab groups. Only one serious TEAE (juvenile angiofibroma) was reported in vunakizumab 120 mg group during the 12 weeks of treatment, but it was not deemed to be treatment-related.
Conclusion: Vunakizumab demonstrated superior efficacy to placebo and was well tolerated with an acceptable safety profile in patients with active PsA.
To cite this abstract in AMA style:
Zou H, Wan W, Xue Y, Sun L, Zhang N, Chen H, Shi X, Liu S, Chen L, Ma X, Wei H, Jiang Z, Li X, Fan H, Li H, Li J, Wu R, Shi G, Zhu J, Kong X, Lu Y, Cui X, Zheng Q, Bai X, Dong G. Vunakizumab in Patients with Active Psoriatic Arthritis: A Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 2 Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/vunakizumab-in-patients-with-active-psoriatic-arthritis-a-multicenter-randomized-double-blind-placebo-controlled-phase-2-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vunakizumab-in-patients-with-active-psoriatic-arthritis-a-multicenter-randomized-double-blind-placebo-controlled-phase-2-study/