Session Information
Date: Tuesday, November 10, 2015
Title: Osteoporosis and Metabolic Bone Disease - Clinical Aspects and Pathogenesis
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
Antiretroviral regimens containing tenofovir disoproxil fumarate (TDF) have been associated with decreases in bone mineral density, and elevations in bone turnover markers (BTM) and intact parathyroid hormone (iPTH) in patients with HIV. Prior cross-sectional studies suggested that a functional vitamin D deficiency may in part explain these changes. To explore this hypothesis further, we measured change in plasma vitamin D binding protein (DBP) levels from baseline to 48 weeks among a cohort of patients treated with TDF/lamivudine(3TC)/efavirenz(EFV) in the context of other serologic markers of vitamin D and bone metabolism.
Methods:
We performed a secondary analysis using plasma samples collected at 0, 24, and 48 weeks after initiation of TDF/3TC/EFV from 140 adult participants enrolled in a multi-center randomized trial. Women over 45 years were excluded to avoid confounding due to menopausal status. Data regarding socio-demographic characteristics, BMI, CD4+ counts, and HIV viral load were obtained as part of the parent study. Laboratory tests included plasma DBP, iPTH, total 25-hydroxyvitamin D (25OHD), the bone resorption marker collagen type 1 cross-linked C-telopeptide (CTX), and the bone formation marker total procollagen type 1 N-terminal propeptide (P1NP). Differences between time points were compared using the paired t-test and Wilcoxin signed-rank test.
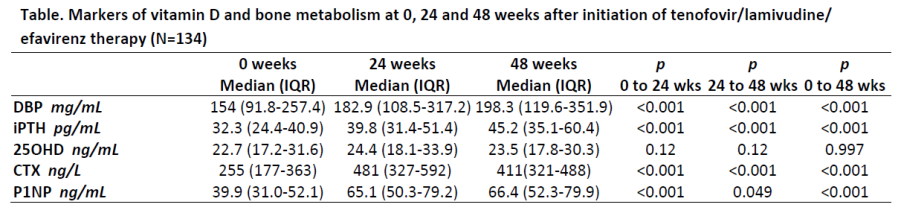
Results:
Our sample included 108 men and 26 women with a mean age of 33.6±9.6 years and BMI of 22.3±2.9 kg/m2. Mean BMI remained stable from 0 to 48 weeks (p=0.47), however median CD4+ count increased significantly from 290.5 (IQR: 201-362) to 424 (IQR: 294-555) cells/mm3 (p<0.001) and median viral load decreased from 53767 (IQR: 19802 to 136493) to 0 (IQR:0 to 10) copies/mL (p<0.001). Significant increases were observed in DBP levels from 0 to 24 weeks followed by smaller increases from 24 to 48 weeks (see Table). Similar increases were detected in iPTH levels, however 25OHD levels remained stable. BTM levels increased significantly from 0 to 24 weeks followed by a slight decline (CTX) or stabilization (P1NP), however remained significantly higher compared with baseline at 48 weeks.
Conclusion:
Plasma levels of DBP rose significantly in the first 24 weeks after initiation of TDF/3TC/EFV, followed by a more modest increase from 24 to 48 weeks. This change was observed in concert with elevations iPTH and BTMs, despite stable 25OHD levels, supporting a potential role for DBP in bone loss associated with TDF therapy.
To cite this abstract in AMA style:
Hsieh E, Fraenkel L, Xia W, Han Y, Yin M, Insogna K, Zhu T, Li T. Vitamin D Binding Protein and Tenofovir-Associated Bone Loss Among Individuals with HIV [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/vitamin-d-binding-protein-and-tenofovir-associated-bone-loss-among-individuals-with-hiv/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vitamin-d-binding-protein-and-tenofovir-associated-bone-loss-among-individuals-with-hiv/